Membrane Rearrangements in Developmental Fusion and Cancer Progression

- Leonid V. Chernomordik, PhD, Head, Section on Membrane Biology
- Eugenia Leikina, DVM, Senior Research Assistant
- Kamram Melikov, PhD, Staff Scientist
- Elena Zaitseva, PhD, Staff Scientist
- Berna Uygur, PhD, Postdoctoral Intramural Research Training Award Fellow
- Jarred Whitlock, PhD, Intramural Research Training Award Fellow
- Gracia Luoma-Overstreet, BS, Postbaccalaureate Fellow
Diverse biological processes, in which enveloped viruses infect cells and cells from all kingdoms of life secrete, internalize, traffic, and sort integral proteins, sculpt their membranes, and bring together parent genomes in sexual reproduction, share a common stage: fusion of two membranes into one. Biological membrane remodeling is tightly controlled by protein machinery but is also dependent on the lipid composition of the membranes. Whereas each kind of protein has its own personality, membrane lipid bilayers have rather general properties manifested by their resistance to disruption and bending and by their charge. Our long-term goal is to understand how proteins fuse membrane lipid bilayers. We expect a better understanding of important fusion reactions to bring about new ways of controlling them and to lead to new strategies for quelling diseases involving cell invasion by enveloped viruses and defects in intracellular trafficking or intercellular fusion. Our general strategy is to combine in-depth analysis of the best characterized fusion reactions with comparative analysis of diverse, less explored fusion reactions, a strategy that can reveal new kinds of fusion proteins and clarify the generality of emerging mechanistic insights. In our recent studies, we focused on the role of the fusion of cancer cells in cancer progression.
Interactions with muscle cells boost fusion, stemness, and drug resistance of prostate cancer cells.
Mechanisms by which interactions between cancer cells and non-malignant cells within the tumor microenvironment influence cancer progression and metastasis are still not understood. In the early 1900’s, Otto Aichel suggested that the leukocyte-like characteristics of metastatic cancer cells , which facilitate their migration through the blood, are acquired by their fusion with white blood cells [Aichel O. Vorträge und Aufsätze Über Entwicklungsmechanik der Organismen. Leipzig, Germany: Wilhelm Engelmann 1911;92-111]. The hypothesis that cell fusion contributes to initiation and progression of cancer and, specifically, aneuploidy, drug resistance, and metastatic potential characteristic of malignant cells has been further developed in several recent studies [reviewed in Reference 2 and by Noubissi FK and Ogle BM Int J Mol Sci 2016;17:pii e1587].
In our recent study [Reference 3], we focused on the role of cell fusion in prostate cancer. The prostate gland is surrounded by the smooth muscle of the prostate stroma and the striated muscle fibers of the rhabdosphincter. While both smooth and skeletal muscle cells are known to produce and secrete many signaling molecules, the effects, if any, of the muscle cells on the development and progression of primary prostate tumors are yet unexplored. In the study, we modeled interactions between cancer cells and muscle cells in in vitro co-cultures and found that primary smooth or skeletal muscle cells increase clonogenic potential and drug resistance of the primary prostate cancer cells and PC3 cells. These interactions also expanded the fraction of the cancer cells expressing the stem-cell marker CD133. We dissected the pathway of these muscle cell–induced changes in the properties of the cancer cells into several distinct steps. First, the interactions of the cancer cells with muscle cells upregulate expression and secretion of interleukins IL-4 and IL-13. The cytokines share receptors and have been linked in earlier studies to both prostate cancer progression and fusion of muscle cells [reviewed in Reference 3]. Second, the cytokines upregulate expression of the fusogens syncytin 1 and annexin A5 [Reference 3]. Third, upregulation of these proteins promotes efficient fusion of the cancer cells with 10–20% of cell nuclei located in fusion-generated multinucleated cells, facilitating the analysis of the underlying mechanisms [Reference 3].
Fusion between cancer cells and fusion between cancer cells and muscle cells is associated with upregulation of syncytin 1 and annexin A5 and is inhibited by blocking their expression. The case for the direct involvement in cancer cell fusion is especially strong for syncytin 1, as blocking fusogenic refolding of syncytin 1 with a peptide inhibitor abolishes fusion [Reference 3]. Suppressing either the activity of Il-4/IL-4 cytokines or cell fusion blocks the increases in the stemness and drug resistance of the cancer cells co-cultured with muscle cells. On the other hand, the effects of the muscle cells can be reconstituted by either treating cancer cells with recombinant IL-4 and IL-13 or by fusing the cancer cells with heterologously expressed syncytin 1. Our work is the first to show that interactions with primary smooth and skeletal muscle cells promote changes in the properties of prostate cancer cells that are consistent with their malignant progression. Furthermore, our study identifies three required and sufficient steps in the underlying pathway and thus provides definitive mechanistic insights into discovered phenomenon. Our finding that, in the human prostate tissue microarrays, prostate cancer cells have higher levels of syncytin 1 and annexin A5 expression than non-malignant tissues suggests the importance of syncytin 1– and annexin A5–dependent cancer cell fusion in the cancer progression in vivo. This novel pathway of microenvironment-driven progression of prostate cancer identified in our in vitro experiments and in vivo analysis (Figure 1) represents a novel paradigm of cancer progression and may present new therapeutic targets. Furthermore, the work, as well as our earlier studies on cell fusion stages in muscle and bone remodeling [References 1 and 4], has emphasized the dependence of multistep fusion pathway on the activity of many proteins, including annexins and syncytin.
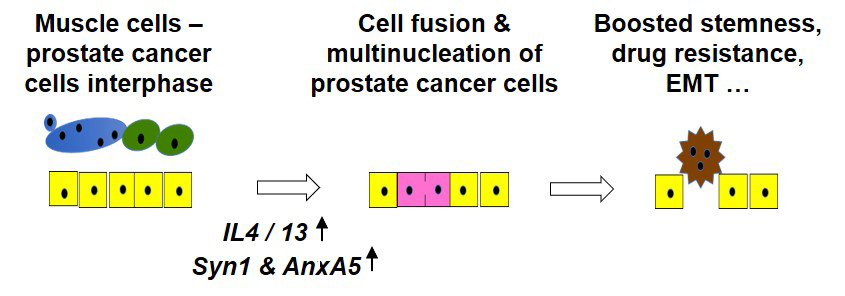
Click image to view.
Figure 1. Suggested pathway by which muscle cells in the microenvironment of prostate cancer cells promote its progression
We propose that interactions of the cancer cells (yellow shapes) with smooth and skeletal muscle cells (green and blue shapes) promote the epithelial-mesenchymal transition (EMT), yielding more invasive mesenchymal cells, and expand the subpopulations of cancer stem-like cells. Such changes in the properties of cancer cells depended on: (1) the muscle cell–induced increases in the concentrations of interleukins 4 and 13 (IL-4/IL-13); (2) the cytokine-induced upregulation of the expression of Syncytin 1 and Annexin A5; and (3) Annexin A5– and Syncytin 1–dependent cancer-cell fusion, which generates multi-nucleated cancer cells (pink shapes), developing the features characteristic of the cancer stem-like cells, including elevated expression of the stem-cell marker CD133, anchorage-independent growth, and drug resistance.
Additional Funding
- United States–Israel Binational Science Foundation (BSF) grant “Machinery of myoblast fusion” 2015–2019
- NICHD Director's Awards, 2018, 2019
- Office of Aids Research Award, 2019, 2020
- NICHD Zika Research Award, 2019
Publications
- Leikina E, Gamage DG, Prasad V, Goykhberg J, Crowe M, Diao J, Kozlov MM, Chernomordik LV, Millay D. Myomaker and Myomerger work independently to control distinct steps of membrane remodeling during myoblast fusion. Dev Cell 2018;46:767–780.
- Brukman NG, Uygur B, Podbilewicz B, Chernomordik LV. How cells fuse. J Cell Biol 2019;218:1436–1451.
- Uygur B, Leikina E, Melikov K, Villasmil R, Verma SK, Vary CPH, Chernomordik LV. Interactions with muscle cells boost fusion, stemness, and drug resistance of prostate cancer cells. Mol Cancer Res 2019;17:806–820.
- Verma SK, Leikina E, Melikov K, Gebert C, Kram V, Young MF, Uygur B, Chernomordik LV. Cell-surface phosphatidylserine regulates osteoclast precursor fusion. J Biol Chem 2018;293:254–270.
Collaborators
- Anush Arakelyan, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Michael M. Kozlov, PhD, Dhabil, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- Leonid Margolis, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Douglas Millay, PhD, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
- Benjamin Podbilewicz, PhD, Technion-Israel Institute of Technology, Haifa, Israel
Contact
For more information, email chernoml@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/chernomordik.


