Molecular Mechanisms of Synapse Assembly, Plasticity, and Homeostasis

- Mihaela Serpe, PhD, Head, Section on Cellular Communication
- Peter Nguyen, Biological Laboratory Technician
- Tae Hee Han, PhD, Staff Scientist
- Saumitra Dey Choudhury, PhD, Visiting Fellow
- Lindsey Friend, PhD, Visiting Fellow
- Wen Chieh Hsieh, PhD, Visiting Fellow
- Tho Huu Nguyen, PhD, Visiting Fellow
- Rosario Vicidomini, PhD, Visiting Fellow
- Thomas Brody, PhD, Special Volunteer
The purpose of our research is to understand the mechanisms of synapse assembly, plasticity, and homeostasis. The chemical synapse is the fundamental communication unit connecting neurons in the nervous system to one another and to nonneuronal cells and whose purpose is to mediate rapid and efficient transmission of signals across the synaptic cleft. Synaptic transmission forms the basis of the biological computations that underlie and enable our complex behavior. Crucial to this function is the ability of a synapse to change its properties, so that it can optimize its activity and adapt to the status of the cells engaged in communication and/or to the larger network comprising them. Consequently, synapse development is a highly orchestrated process coordinated by intercellular communication between the pre- and postsynaptic compartments and by neuronal activity itself. Our long-term goal is to elucidate the molecular mechanisms that regulate formation of functional synapses during development and which fine-tune them during plasticity and homeostasis. We focus on four key processes in synaptogenesis: (1) trafficking of components to the proper site; (2) organizing those components to build synaptic structures; (3) maturation of the synapse to optimize its activity; and (4) homeostatic mechanisms that restore synapse activity after various perturbations in the system. We address the molecular mechanisms underlying these processes using a comprehensive set of approaches that include genetics, biochemistry, molecular biology, superresolution imaging, and electrophysiology recordings in live animals and reconstituted systems.
Because of its many advantages, we choose to study these events in a powerful genetics system, Drosophila melanogaster, and to use the neuromuscular junction (NMJ) as a model for glutamatergic synapse development and function. The fact that individual NMJs can be reproducibly identified from animal to animal and are easily accessible for electrophysiological and optical analysis makes them uniquely suited for in vivo studies on synapse assembly, growth, and plasticity. In addition, the richness of genetic manipulations that can be performed in Drosophila permits independent control of individual synaptic components in distinct cellular compartments. Furthermore, the fly NMJ relies entirely on kainate receptors, which impact synaptic transmission and neuronal excitability in the mammalian central nervous system but remain poorly understood. The Drosophila NMJ can thus be used to analyze and model defects in the structural and physiological plasticity of glutamatergic synapses, which are associated with a variety of human pathologies, from learning and memory deficits to autism. The similarity in architecture, function, and molecular machinery supports the notion that studying the assembly and development of fly glutamatergic synapses will shed light on their human counterparts.
Neto, an essential protein that recruits neurotransmitter receptors and organizes postsynaptic densities at the Drosophila NMJ
Many neurological disorders are linked to defects in synaptogenesis. The initial clustering functions of receptors in synaptogenesis are poorly understood. Prior to motor neuron arrival at its target muscle, the ionotropic glutamate receptors (iGluRs) form small, nascent clusters on the muscle, which are distributed in the vicinity of future synaptic sites. Neuron arrival triggers formation of large synaptic iGluR aggregates and promotes expression of more iGluRs to permit synapse maturation and growth. The iGluR clusters interact with the local cytoskeleton and other synaptic structures to maintain local density, which involves solving two fundamental problems common to all chemical synapses: (1) trafficking the components to the proper site; and (2) organizing those components to build synaptic structures. Recent advances, particularly from vertebrate iGluR biology, reveal that the solution to these problems is entirely dependent on the activity of a rich array of auxiliary subunits that associate with the receptors. These highly diverse transmembrane proteins associate with iGluRs at all stages of the receptor life-cycle and mediate the delivery of receptors to the cell surface, their distribution, synaptic recruitment, association with various postsynaptic density (PSD) scaffolds, and importantly, their channel properties. iGluRs assembled from different subunits have strikingly different biophysical properties; their association with different auxiliary subunits increases this diversity even further.
The Drosophila NMJ utilizes at least six kainate receptor (KAR) subunits, which form two distinct postsynaptic complexes (type-A and type-B) that co-exist within individual PSDs and enable NMJ functionality and plasticity and a presynaptic KAR (KaiRID)–containing complex that modulates basal neurotransmission. The postsynaptic KARs are heterotetrameric complexes composed of three shared subunits, GluRIIC, GluRIID, and GluRIIE, and either GluRIIA (type-A receptors) or GluRIIB (type-B). The shared subunits are essential for viability and for iGluR synaptic recruitment. Our previous studies identified Drosophila Neto as an obligatory subunit of the fly NMJ iGluR complexes. Neto belongs to a family of highly conserved auxiliary proteins that share an ancestral role in the formation and modulation of glutamatergic synapses. Vertebrate Neto1 and Neto2 and Caenorhabditis elegans Neto/SOL-2 were recently shown to modulate the properties of selective iGluRs, mostly KARs. Neto1/Neto2 double knockout mice have defects in long-term potentiation and in learning and memory, but the underlying mechanisms are extremely difficult to study owing to the low abundance of these channels and the small currents they elicit. In contrast, we found that Drosophila utilizes Neto and KARs at the NMJ, a synapse essential for viability. Using live imaging, we showed that Neto clusters at nascent NMJs at the time when iGluRs begin to accumulate and cluster [Kim YJ, et al. Genes Dev 2012;26:974]. Similar to animals lacking essential, shared iGluR subunits, netonul mutants are completely paralyzed and die as embryos, with the iGluRs scattered as small aggregates, away from the neuronal arbor. Importantly, Neto does not cluster at synaptic locations in the absence of iGluRs. Our studies demonstrate that Neto engages the iGluRs on the muscle membrane and that they traffic together to synaptic sites where they form clusters. By controlling the clustering and trafficking of functional iGluR complexes, Neto directly controls synapse assembly, organization and maintenance of PSDs, and synapse functionality [Kim YJ, et al. Genes Dev 2012;26:974; Kim YJ, et al. PLoS Genet 2015;11:e1004988; Kim YJ, Serpe M. Fly (Austin) 2013;7:146; Ramos CI, et al. PLoS Genet 2015;11:e1005191].
Neto-mediated intracellular interactions sculpt the postsynaptic iGluR fields.
The Neto proteins are multidomain transmembrane proteins with two extracellular CUB (for complement C1r/C1s, UEGF, BMP-1) domains followed by an LDLa (low-density lipoprotein receptor domain class A) motif. CUB domains are BMP–binding, protein-interaction domains that could promote aggregation. Drosophila neto encodes two isoforms, Neto-α and Neto-β, with different cytoplasmic domains generated by alternative splicing. The cytoplasmic domains, both rich in putative phosphorylation motifs and docking sites, are highly divergent in Neto proteins across species, presumably reflecting cell- and/or tissue-specific roles. To characterize the functional domains of Neto, we generated truncated Neto variants and tested their cellular distribution and ability to rescue Neto function during development. We found that the extracellular part of Neto is required for apical targeting as well as for clustering of Neto/iGluR complexes at the NMJ. Muscle expression of a Neto variant with no intracellular domain (Neto-ΔCTD) can rescue the iGluRs recruitment in netonull mutants. Neto activities are restricted by an inhibitory prodomain which must be removed by Furin-mediated proteolysis (Kim YJ, et al. PLoS Genet 2015;11:e1004988). When the prodomain cleavage is blocked, Neto is properly targeted to the muscle membrane and engages the iGluR complexes in vivo but fails to enable the incorporation of iGluRs in stable synaptic clusters. The recruitment of PSD components is partly attributable to Neto-mediated intracellular interactions.
Type-A and type-B glutamate receptor complexes differ in their trafficking to the synapses, subsynaptic localization, and synaptic responses; at synapses with both type-A and -B receptors, the dose of GluRIIA vs. GluRIIB is a key determinant of quantal size (the response of the muscle to the spontaneous release of a single synaptic vesicle). Previous work from our lab and others established that the synaptic recruitment of GluRIIA requires several postsynaptic components. Such postsynaptic contribution complements Neto’s ability to retain iGluRs at synaptic sites via extracellular interactions, the “clustering capacity.” We found that mutants with high iGluR “clustering capacity” that lack the relevant postsynaptic interactions cannot stabilize synaptic type-A receptors and incorporate type-B instead. We propose that the type-B receptors are the “default receptors” at synapses with adequate clustering capacity, while the type-A receptors require an extensive network for their synaptic stabilization.
A first evidence in support of this model comes from our studies on Neto-β, the predominant Neto isoform at the larval NMJ (Ramos CI, et al. PLoS Genet 2015;11:e1005191). Our developmental studies indicate that Neto-β controls the synaptic recruitment of iGluRs and of other postsynaptic components, such as P21-activating kinase (PAK), an important PSD component previously implicated in the stabilization of type-A receptors at postsynaptic sites. The neto-βnull synapses have reduced iGluRs synaptic clusters, in particular the type-A subtype; this reflects the drastic reduction of Neto net levels. However, a neto-βshort allele, which truncates part of the cytoplasmic domain and produces a shorter Neto-β variant, shows increased accumulation of synaptic GluRIIB and much reduced GluRIIA compared with control synapses. Thus, short Neto-β, which cannot recruit type-A stabilizers, clusters type-B receptors, suggesting that Neto-β uses its cytoplasmic domain as an organizing platform to sculpt postsynaptic composition.
Interestingly, loss of Neto-α has no detectable effect on iGluR synaptic accumulation [Reference 3]. Instead, 3D-SIM (secondary-ion mass spectrometry) analysis captured the enlarged receptor fields, which appeared to fill the small neto-αnull boutons (Figure 1). Muscle overexpression of a neto-α transgene fully rescued the PSD size of neto-αnull synapses. Thus, Neto-α limits the size of the postsynaptic receptor fields but has no detectable role in the organization of presynaptic specializations.
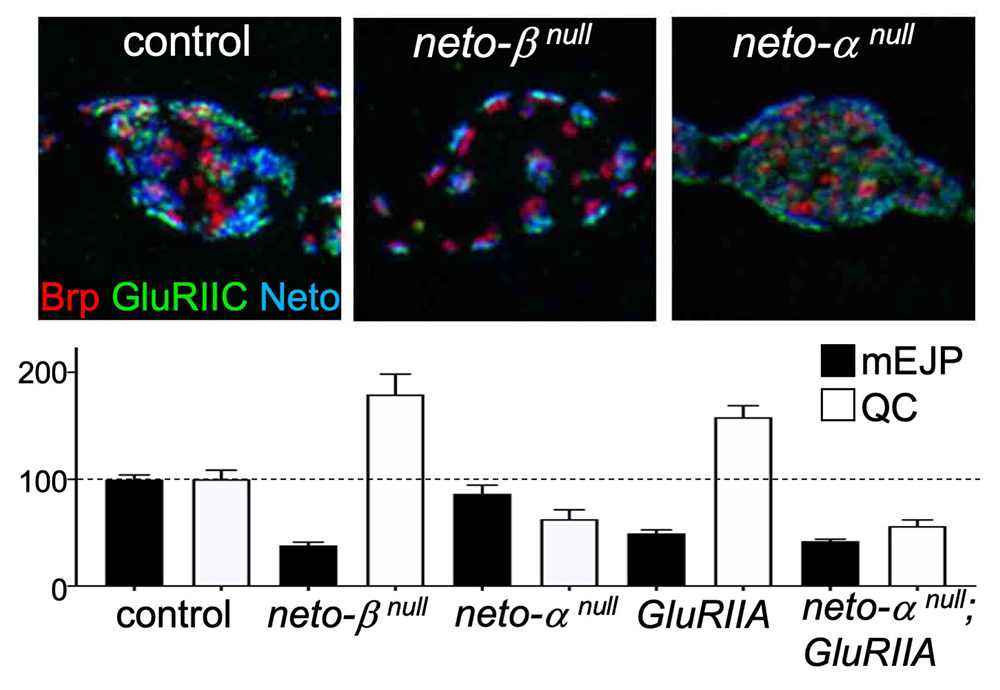
Click image to view.
Figure 1. Neto isoforms have distinct roles in PSD organization (upper) and NMJ function (lower).
3D-SIM images of synaptic boutons stained for the active zone scaffold, Brp (red), GluRIIC (green) and Neto (blue). Individual synapses are much reduced in the absence of Neto-β and enlarged in the absence of Neto-α.
Reduced postsynaptic sensitivities (mEJP) trigger a compensatory increase in quantal content (QC), the number of vesicle released by the neuron in most genotypes, except for neto-αnull.
Neto-α controls basal neurotransmission and synapse homeostasis.
In flies as in vertebrates, neuronal activity induces input-specific changes in the synaptic strength; at the larval NMJ, the postsynaptic sensitivity is primarily modulated by synapse-specific recruitment of type-A (GluRIIA-containing) receptors. Robust homeostatic mechanisms keep synapses within an appropriate dynamic range, so that the evoked potentials measured in the muscle remain constant from embryo to third instar larvae; reduced postsynaptic sensitivities (i.e., reduced GluRIIA activity) trigger a compensatory increase in quantal content (QC), the number of vesicle released by the neuron, referred to as presynaptic homeostatic potentiation (PHP).
The drastic reduction of synaptic iGluRs (primarily GluRIIA) at neto-βnull NMJs causes reduced mini frequency and amplitudes (mEJP), but these NMJs have normal evoked potentials owing to increased QC [Ramos CI, et al. PLoS Genet 2015;11:e1005191] and (Figure 1). Neto-α accounts for less than 10% of the Neto synaptic pool. Nonetheless, neto-αnull NMJs have normal mini amplitudes, but reduced basal neurotransmission [Reference 3]. Interestingly, neuronal but not muscle expression of a neto-α transgene rescues the basal neurotransmission, indicating that Neto-α functions in the presynaptic compartment to modulate basal neurotransmission. We examined the homeostatic responses at neto-αnull NMJs using well-established paradigms, including chronic (developmental) and acute (pharmacological) induction of PHP. Loss of presynaptic Neto-α renders these NMJs unable to express PHP [Reference 3]. Specifically, (1) removal of GluRIIA during development leads to reduced quantal size (mEJP) and triggers PHP (increased QC), a PHP response that is not detectable in neto-αnull;GluRIIA double mutants (Figure 1); also, (2) application of sub-blocking concentrations of philanthotoxin (PhTx), a polyamine toxin derived from wasp venom, to semi-intact larval preparations triggers a fast reduction in quantal size and an increase in QC, so that the basal neurotransmission recovers within minutes. PhTx reduces the quantal size at neto-αnull NMJs, but the basal neurotransmission never recovers.
Given that a presynaptic KAR, KaiRID, has been recently implicated in the control of basal neurotransmission and the expression of PHP [Kiragasi B, et al. Cell Rep 2017;19:2694], we examined whether Neto-α modulates KaiRID synaptic distribution and function. We found that Neto-α controls neurotransmitter release in a KAR–dependent manner. Furthermore, Neto-α is both required and sufficient for the PHP response, which includes an expansion of the vesicle release machinery. Interestingly, neuronal expression of Neto-β cannot rescue neto-αnull PHP deficits because Neto-β cannot traffic to the synaptic terminals and instead remains restricted to the somato-dendritic compartment. In contrast, a Neto variant with no intracellular domains (Neto-ΔCTD) can reach the presynaptic terminal and rescue the basal neurotransmission defects of neto-αnull, but cannot restore the PHP. Our studies demonstrate that the intracellular part of Neto-α functions as a bona fide effector of PHP. The limiting Neto-α seems to be recruited at synapses by the presynaptic KaiRID. This finding challenges our current thinking that auxiliary subunits “assist” iGluRs, and it provides an exquisite example of an auxiliary protein that performs a key synaptic function with assistance from iGluRs.
Our current efforts focus on identifying proteins that interact with Neto both inside and outside the cell and that provide critical activities at the developing NMJ, including iGluR–clustering, iGluR-recruitment and stabilization at PSDs, and mediation of PHP. To this end, we initiated complementary screens: pull-down and mass-spectroscopy comparisons of proteins interacting with the intracellular domains of the fly Neto proteins (α and β); and a synthetic lethality screen (see below).
Tenectin, an integrin ligand critical for structural and functional integrity of the fly NMJ
To search for novel extracellular matrix proteins important for NMJ development, we took advantage of neto109, a strong neto hypomorph mutant that we isolated and characterized in our lab. The mutant has drastically diminished levels of synaptic iGluRs, but normal net levels of muscle receptors, indicating a defect in the trafficking and/or stabilization of receptors at junctional locations. Given that 50% of neto109 hypomorphs die during development, further reduction of synaptogenic proteins in hemizygous animals should increase lethality. Using this rationale, we set up a synthetic lethality screen to identify proteins that interact genetically with Neto and that control the development of NMJ. In a pilot, proof-of-concept screen of candidate interactors, we confirmed known NMJ modulators, such as Glass bottom boat (Gbb), a BMP ligand with critical roles during NMJ development. In a search for ECM (extracellular matrix) candidates, we identified a set of overlapping deficiencies that dramatically increased the lethality of neto109 hemizygotes. Among the common loci disrupted by these deficiencies was tenectin (tnc), a gene encoding a large, secreted protein conserved in many insects but with no obvious mammalian homolog.
Tnc is a large secreted mucin that forms a gel-like structure used as luminal scaffolds during the development of tubular epithelia. We found that Tnc is also secreted from motor neurons and muscles and accumulates in the synaptic cleft at larval NMJ. Reduction of Tnc via RNAi produced flightless adults with locomotor defects. To examine the role of tnc in synapse development, we generated tnc alleles and found that tnc is essential for normal NMJ morphology and function. Tnc–deprived NMJs have small, distorted boutons and reduced probability of vesicle release. Interestingly, Tnc recruits αPS2/βPS integrin at synaptic terminals, but not at the muscle attachment sites. Such selectivity was instrumental in probing for integrin functions during synapse development. We found that Tnc and αPS2/βPS integrin form cis-active complexes with distinct pre- and postsynaptic functions. In muscle, Tnc/integrin regulates the size and architecture of synaptic boutons, partly through recruitment of the spectrin-based membrane skeleton. In motor neurons, Tnc/integrin ensures the proper assembly and function of active zones by recruiting key players in neurotransmitter release, including the voltage-gated Ca2+ channel Cacophony (Cac), and the active-zone scaffold Bruchpilot (Brp). Neuronal knockdown of α-spectrin also diminished synaptic Cac and Brp, prompting us to examine whether Tnc recruits presynaptic spectrin.
A presynaptic spectrin network controls active zone assembly and neurotransmitter release.
To detect neuronal α-Spectrin, we employed a GFP reconstitution approach whereby one fragment of the split GFP, spGFP11, was introduced into the α-Spectrin locus via CRISPR/Cas9, and the second part, GFP1-10, was expressed intracellularly. In motor neurons, the GFP–marked lattice was bright along the axons, and moderate from the point where motor neuron arbors sink into the muscle cortex (Figure 2). Autocorrelation analyses indicated that the lattice spacing was about 200 nm, consistent with previous measurements. Within the synaptic terminal, the GFP–marked α-Spec fanned out and filled the synaptic boutons but coalesced into a bundle in the interbouton regions. In the absence of Tnc, the GFP–marked α-Spec remained bright along the axons but was drastically diminished at the synaptic terminals. Interestingly, we found that presynaptic α-Spectrin is both required and sufficient for the recruitment of Cac and Brp. We propose that Tnc/integrin complexes anchor the presynaptic spectrin network and ensure proper assembly and function of active zones. Together with our previous finding that postsynaptic Tnc/integrin recruits spectrin to modulate the structural integrity of synaptic boutons, our work identifies two spectrin-dependent integrin signaling pathways that coordinate synapse development and function.
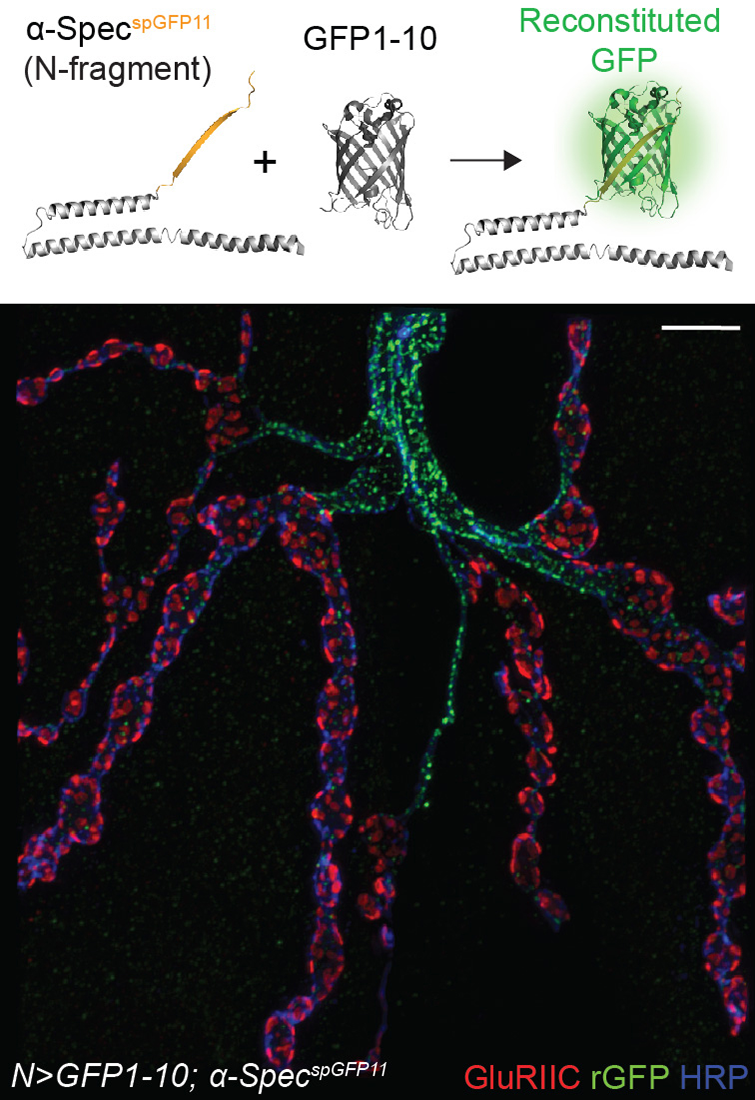
Click image to view.
Figure 2. Reconstituted GFP (rGFP) marks the endogenous spectrin lattice in larval motor neurons.
3D-SIM image of a motor neuron terminal stained for rGFP, GluRIIC, and HPR (which labels the neuronal membrane).
Integrin activation generally promotes formation of focal adhesion complexes and cell migration. In the presence of Tnc, S2 cells (insect cells) accumulate integrin and spectrin at their membranes, but they neither migrate nor form focal adhesion points; instead, the cells round up. This is reminiscent of erythrocytes that maintain their shape and function through the membrane-associated skeleton. Several patients with acanthocytosis (spiky erythrocytes) caused by spectrin tetramerization deficits also present nervous system abnormalities, underscoring the relevance of spectrin network for normal neuronal development and function. Our studies reveal how dynamic changes in the extracellular matrix could be transduced via ligand-activated integrin and spectrin to coordinate changes in synapse structure and function.
Local BMP/BMPR complexes regulate synaptic plasticity.
Synaptic activity and synapse development are intimately linked, but our understanding of the coupling mechanisms is limited. In particular, how synapse activity status is monitored and communicated across the synaptic cleft remains poorly understood. Our studies uncovered a role for bone morphogenetic proteins (BMPs) in sensing the activity of postsynaptic receptors and relaying this information across the synaptic cleft.
At the Drosophila NMJ, BMP signaling is critical for NMJ growth, neurotransmitter release, and synapse plasticity and homeostasis. Our work uncovered a novel BMP signaling modality that operates in conjunction with the canonical BMP pathway to ensure these functions. The canonical BMP pathway, triggered by muscle-derived Gbb binding to presynaptic BMP type-II receptor (BMPRII), Wishful thinking (Wit), BMPRIs Thickveins (Tkv), and Saxophone (Sax), induces accumulation of phosphorylated Smad (pMad) in motor neuron nuclei and activates transcriptional programs with distinct roles in the structural and functional development of the NMJ. Gbb and Wit also signal noncanonically through the effector protein LIM kinase 1 (LIMK1) to regulate synapse stability. Interestingly, pMad also accumulates at synaptic locations but the biological relevance of this phenomenon remained a mystery for over a decade. We found that synaptic pMad constitutes a sensor for synapse activity [Sulkowski M, et al. Development 2014;141:436]. Furthermore, synaptic pMad marks a novel, noncanonical BMP pathway, genetically distinguishable from all other known BMP signaling cascades [Reference 4; Sulkowski M, et al. PLoS Genet 2016;12:e100581]. This novel pathway stabilizes postsynaptic type-A (GluRIIA–containing) glutamate receptors as a function of their activity.
Type-A receptors are the first to arrive at a nascent synapse; they form the “core” of receptor field and are surrounded by type-B receptors. Previous work in our lab and others established that the incorporation of type-A in stable synaptic complexes depends on GluRIIA activity and requires an extensive postsynaptic network. Our studies on the novel BMP signaling modality indicate that the synaptic stabilization of type-A receptors also requires transsynaptic complexes. The question arises as to how postsynaptic glutamate receptors modulate presynaptic pMad and are in turn stabilized by it. Given that synaptic pMad depends on active type-A receptors, we favor a model whereby Neto, via its BMP-binding CUB domains, connects active postsynaptic type-A receptors with presynaptic BMP/BMPR complexes (Figure 3). Such transsynaptic complexes could offer a versatile means for relaying synapse activity status to the presynaptic neuron via fast conformational modifications. At the same time, these transsynaptic complexes may facilitate interactions that stabilize the type-A receptors at the PSD, a positive feedback that could explain the Hebbian mode of GluRIIA incorporation at the PSD and maturation of iGluR fields at larval NMJ. As BMPRs are limiting and shared among different BMP signaling modalities, the neurons may use this novel BMP pathway to monitor synapse activity and then coordinate NMJ growth with synapse maturation and stabilization.
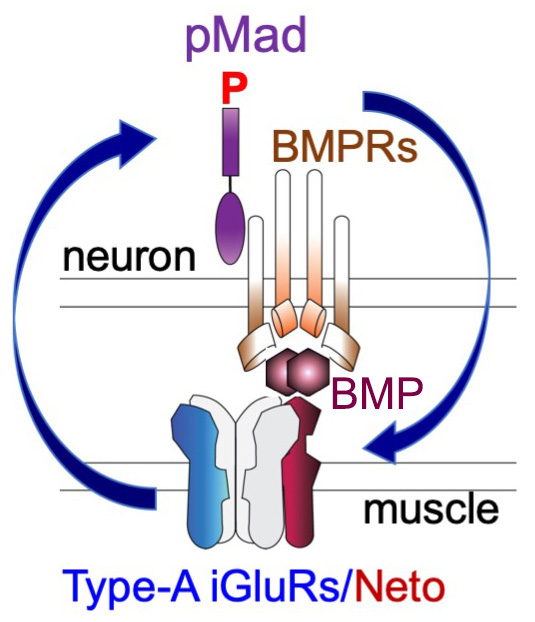
Click image to view.
Figure 3. The positive feedback loop model
Neto, via its BMP–binding CUB domains, connects active postsynaptic type-A channels with presynaptic BMP/BMP Receptor complexes. Active type-A channels trigger accumulation of presynaptic BMP/BMPR complexes (marked by pMad), which in turn stabilize the type-A channels at postsynaptic sites.
Selective disruption of synaptic BMP signaling by a Smad mutation adjacent to the conserved H2 helix
In search of Mad features that influence its association with the BMPRs, we collected most existing Drosophila Mad alleles and compared them for their ability to sustain the two Smad–dependent signaling modalities: canonical BMP signaling, marked by pMad accumulation in motor neuron nuclei, and Smad–dependent noncanonical signaling, marked by pMad accumulation at synaptic terminals. Within this comprehensive collection, we found that strong Mad alleles generally disrupt both synaptic and nuclear pMad accumulation, whereas moderate Mad alleles have a wider range of phenotypes and selectively impact different BMP signaling modalities. In particular, Mad8 showed drastically reduced synaptic pMad levels but only moderately diminished nuclear pMad signals. The postsynaptic composition and electrophysiological properties of Mad8 NMJs were likewise altered. Using biochemical assays and structural modeling, we examined how point mutations such as S359L (marked*, Figure 4), present in Mad8, could influence the Mad–Tkv interface. Our study identified a new molecular determinant for this Mad–Tkv interaction, the highly conserved H2 helix [Reference 4]. Several genetic variants identified in human patients map to H2, underscoring the relevance of this motif for normal development and function.
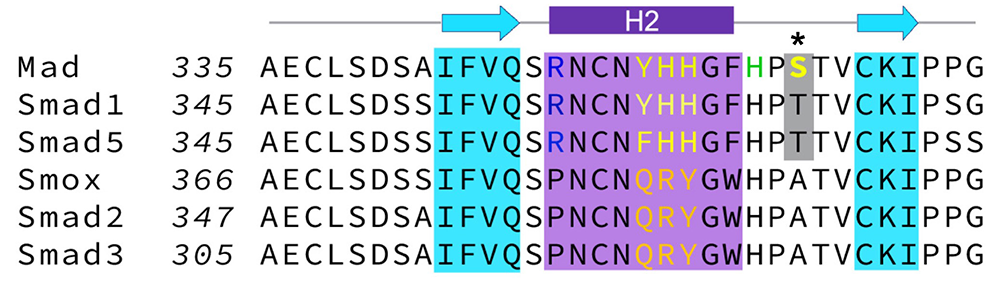
Click image to view.
Figure 4. The H2 helix is a critical molecular determinant for the Smad–BMPRI interaction.
Alignment indicating class-specific residues in the H2 helix: Y352HH in Smads of the BMP pathway, and QRY in the equivalent position in Smads of the Activin pathway.
Additional Funding
- NICHD Director’s Challenge Award Program in collaboration with Mary Dasso (NICHD) and Brian Oliver (NIDDK).
Publications
- Wang Q, Han TH, Nguyen P, Jarnik M, Serpe M. Tenectin recruits integrin to stabilize bouton architecture and regulate vesicle release at the Drosophila neuromuscular junction. eLife 2018;7:e35518.
- Wang Q, Friend L, Vicidomini R, Han TH, Nguyen P, Ting C-Y, Serpe M. A presynaptic spectrin network controls active zone assembly and neurotransmitter release. bioRxiv 2019;https://doi.org/10.1101/812032.
- Han TH, Vicidomini R, Ramos CI, Wang Q, Nguyen P, Jarnik M, Li M, Sawarski M, Hernandez RX, Macleod GT, Serpe M. Neto-alpha controls synapse organization and homeostasis at the Drosophila neuromuscular junction. bioRxiv 2019;https://doi.org/10.1101/812040.
- Nguyen TH, Han TH, Newfeld S, Serpe M. Selective disruption of synaptic BMP signaling by a Smad mutation adjacent to the highly conserved H2 helix. bioRxiv 2019;https://doi.org/10.1101/811109.
- Arnaoutov A, Lee HN, Plevock Haase K, Jarnik M, Oliver B, Serpe M, Dasso M. IRBIT directs differentiation of intestinal stem cell progeny to maintain tissue homeostasis. bioRxiv 2019;https://doi.org/10.1101/737262.
Collaborators
- Gregory T. Macleod, PhD, Florida Atlantic University, Jupiter, FL
- Mark Mayer, PhD, Scientist Emeritus, NINDS, Bethesda, MD
- Stuart Newfeld, PhD, School of Life Sciences, Arizona State University, Tempe, AZ
Contact
For more information, email serpemih@mail.nih.gov or visit http://ucc.nichd.nih.gov.


