Cell Cycle Regulation in Oogenesis

- Mary Lilly, PhD, Head, Section on Gamete Development
- Chun-yuan Ting, PhD, Staff Scientist
- Kuikwon Kim, MS, Technician
- Lucia Bettedi, PhD, Visiting Fellow
- Yingbiao Zhang Zhang, PhD, Visiting Fellow
- Shu Yang, PhD, Postdoctoral Fellow
- Christa Ventresca, BS, Postbaccalaureate Fellow
Our long-term goal is to obtain a comprehensive understanding of how metabolic signaling pathways influence oocyte growth, development, and quality. Chromosome mis-segregation during female meiosis is the leading cause of miscarriages and birth defects in humans. Recent evidence suggests that many meiotic errors occur downstream of defects in oocyte growth and/or the hormonal signaling pathways that drive differentiation of the oocyte. Thus, understanding how oocyte development and growth impact meiotic progression is essential to studies in both reproductive biology and medicine. We use the genetically tractable model organism Drosophila melanogaster to examine how meiotic progression is instructed by the developmental and metabolic program of the egg.
In mammals, studies on the early stages of oogenesis face serious technical challenges in that entry into the meiotic cycle, meiotic recombination, and the initiation of the highly conserved prophase I arrest all occur during embryogenesis. By contrast, in Drosophila these critical events of early oogenesis all take place continuously within the adult female. Easy access to the early stages of oogenesis, coupled with available genetic and molecular genetic tools, makes Drosophila an excellent model for studies on the role of metabolism in oocyte development and maintenance.
The GATOR complex: integrating developmental and metabolic signals in oogenesis
The Target of Rapamycin Complex 1 (TORC1) regulates cell growth and metabolism in response to many inputs, including amino acid availability and intracellular energy status. In the presence of sufficient nutrients and appropriate growth signals, the Ragulator and the Rag GTPases (a complex that regulates lysosomal signaling and trafficking) target TORC1 to lysosomal membranes, where TORC1 associates with its activator, the small GTPase Rheb. Once activated, TORC1 is competent to phosphorylate its downstream targets. The Gap Activity Towards Rags (GATOR) complex is an upstream regulator of TORC1 activity.
The GATOR complex consists of two subcomplexes (Figure 1). The GATOR1 complex inhibits TORC1 activity in response to amino acid starvation. GATOR1 is a trimeric protein complex consisting of the proteins Nprl2, Nprl3, and Iml1. Evidence from yeast and mammals indicates that the components of the GATOR1 complex function as GTPase–activating proteins (GAP) that inhibit TORC1 activity by inactivating the Rag GTPases. Notably, Nprl2 and Iml1 are tumor suppressor genes, while mutations in Iml1, known as DEPDC5 in mammals, are a leading cause of hereditary epilepsy.
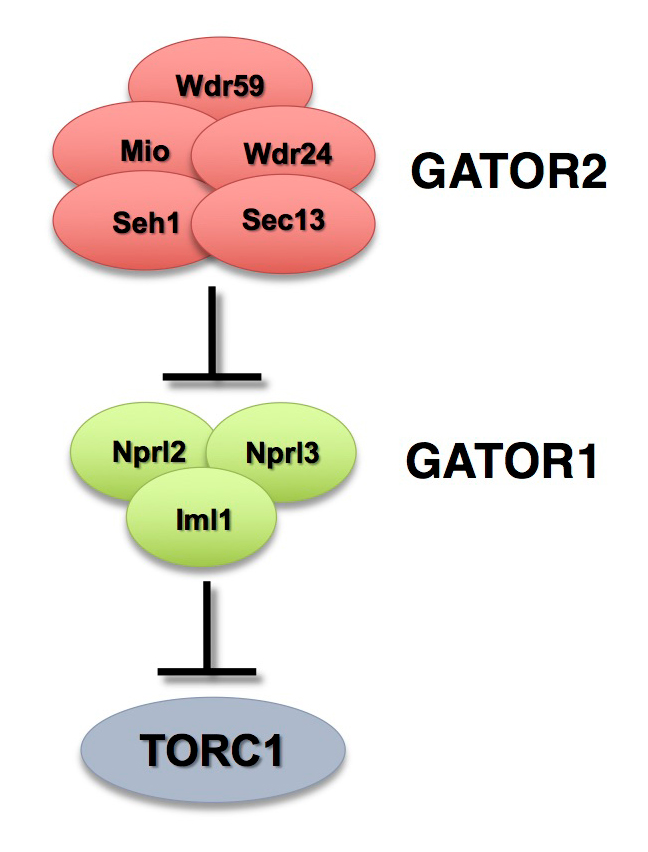
Click image to view.
Figure 1. The GATOR complex regulates TORC1 activity.
The GATOR2 complex opposes the activity of the TORC1 inhibitor GATOR1.
The GATOR2 complex comprises five proteins: Seh1, Sec13, Mio, Wdr24, and Wdr59. Our work, as well as that of others, found that the GATOR2 complex activates TORC1 by opposing the TORC1–inhibitory activity of GATOR1. Intriguingly, computational analysis indicates that Mio and Seh1, as well as several other members of the GATOR2 complex, have structural features consistent with coatomer proteins and membrane-tethering complexes. In line with the structural similarity to proteins that influence membrane curvature, we showed that three components of the GATOR2 complex, Mio, Seh1, and Wdr24, localize to the outer surface of lysosomes, the site of TORC1 regulation. However, how GATOR2 inhibits GATOR1 activity, thus allowing for the robust activation of TORC1, remains unknown. Additionally, the role of the GATOR1 and GATOR2 complexes in both the development and physiology of multicellular animals remains poorly defined. Over the last year, we used molecular, genetic, and cell-biological approaches to define the role of the GATOR complex in the regulation of Drosophila oocyte development and physiology.
The GATOR complex regulates an essential response to meiotic double-stranded breaks.
The TORC1 inhibitor GATOR1 controls meiotic entry and early meiotic events in yeast. However, how metabolic pathways influence meiotic progression in metazoans remains poorly understood. During the last year, we expanded our examination of how the TORC1 regulators GATOR1 and GATOR2 mediate a response to meiotic DNA double-stranded breaks (DSBs) during Drosophila oogenesis. The initiation of homologous recombination through the programmed generation of DNA DSBs is a universal feature of meiosis. DSBs represent a dangerous form of DNA damage, which can result in dramatic and permanent changes to the germline genome. To minimize their destructive potential, the generation and repair of meiotic DSBs is tightly controlled in space and time. We showed that meiotic DSBs promote the GATOR1–dependent down-regulation of TORC1 activity. Consistent with this observation, we found that mutants in genes such as the Rad51 homolog spnA, which retain meiotic DSBs into late stages of oogenesis, exhibit a profound reduction in TORC1 activity in the female germline (Figure 1), data that suggest that low TORC1 activity may be important for the efficient repair of meiotic DSB. In line with this hypothesis, we determined that GATOR1–mutant ovaries, which have high levels of TORC1 activity, exhibit many phenotypes consistent with the misregulation of meiotic DSBs, including an increase in the steady-state number of meiotic DSBs, the retention of meiotic DSBs into later stages of oogenesis, and the hyperactivation of p53, a transcription factor that mediates a highly conserved response to genotoxic stress (Figure 2). Importantly, RNAi depletions of Tsc1 phenocopied the GATOR1 ovarian defects. TSC1 is a component of the potent TORC1 inhibitor Tuberous Sclerosis Complex (TSC), confirming that the misregulation of meiotic DSBs observed in GATOR1–mutant oocytes is attributable to high TORC1 activity rather than to a TORC1–independent function of the GATOR1 complex. Further genetic analysis revealed that many of the phenotypes associated with high TORC1 activity observed in GATOR1–mutant ovaries are the result of the hyperactivation of the downstream TORC1 target S6K. We also demonstrated that GATOR1 impacts the repair, rather than the generation, of meiotic DSBs. Our data are particularly intriguing in light of similar meiotic defects observed in npr3 mutants in Saccharomyces cerevisiae. These results raise the possibility that GATOR1–mediated downregulation of TORC1 activity may be a common feature of the early meiotic cycle in many eukaryotes.
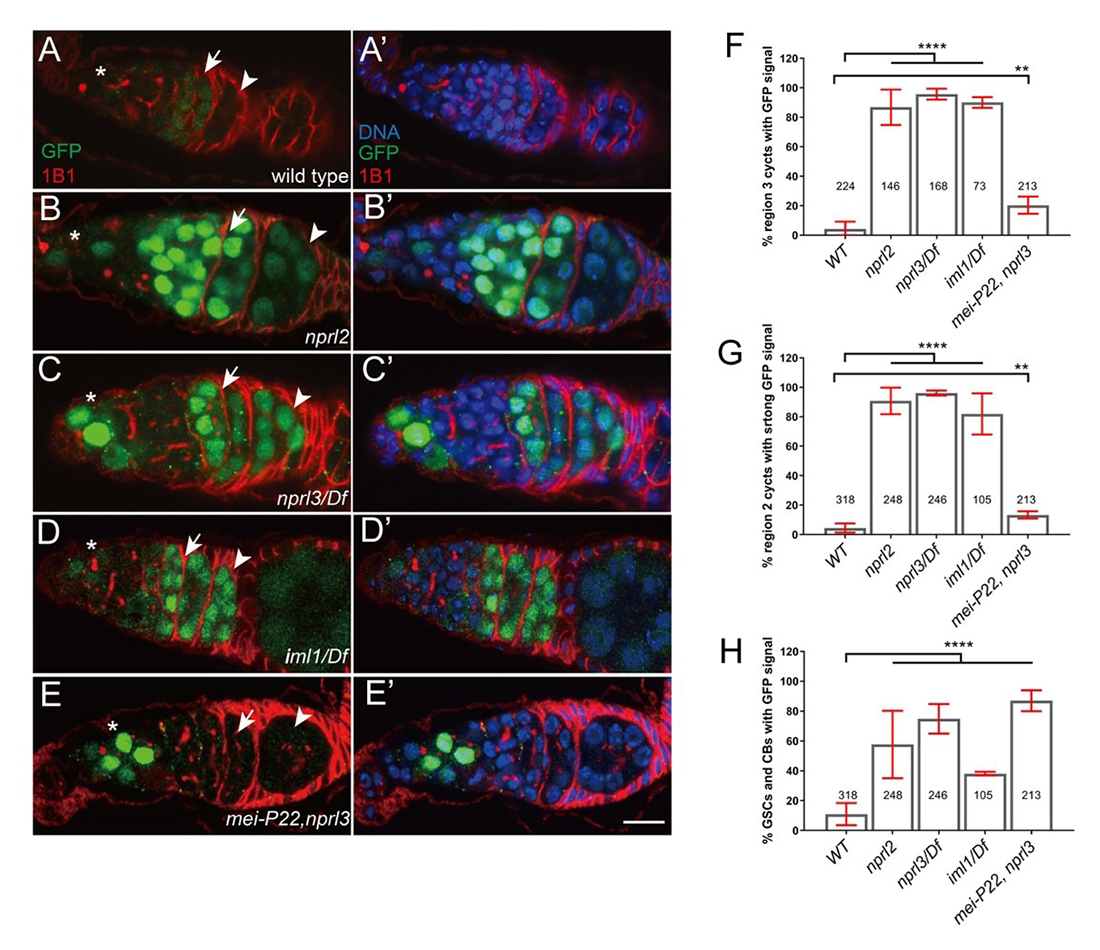
Click image to view.
Figure 2. GATOR1 prevents p53 hyperactivation in Drosophila early ovarian cysts.
Ovaries from (A) p53R–GFP, (B) nprl21; p53R–GFP, (C) p53R–GFP;nprl31/Df, (D) p53R–GFP; iml1/Df, and (E) p53R–GFP; mei-P22p22, nprl31 were stained for GFP (green) and 1B1 (red). Germarial regions are defined by 1B1 staining. In wild-type ovaries the p53–GFP reporter is briefly activated in region 2 (indicated by arrow). Note the low level of GFP staining. In contrast, in GATOR1 mutants, p53R–GFP is robustly activated, with the strong GFP signal often persisting into germarial region 3 and beyond. Additionally, in GATOR1–mutant germaria, p53R–GFP is frequently activated in germline stem cells (GSC) and daughter cystoblasts (CB). In mei-P22p22, nprl31 double mutant germaria, the hyperactivation of p53R–GFP is rescued in region 2a ovarian cysts. However, p53–GFP activation in GSC and CB is retained in the double mutants (asterisk), indicating that in these cells the activation of p53 is not contingent on the presence of meiotic double-stranded breaks (DSBs). Scale bars, 10μm. (F) Percentage of germaria that sustain p53R–GFP signal in region 3. (G) Percentage of germaria with high p53R–GFP signal in region 2. (H) Percentage of germaria with p53R–GFP expression in GSC and CB. Unpaired T-student test was used to calculate the statistical significance. Error bars represent SD from at least three independent experiments. **P < 0.01, ****P < 0.0001.
GATOR1 prevents the derepression of retrotransposons in response to meiotic double-stranded breaks.
Genotoxic stress has been implicated in the deregulation of retrotransposon expression in several organisms, including Drosophila. In line with these studies, we find that, in GATOR1 mutants, the double-stranded breaks that initiate meiotic recombination trigger the deregulation of retrotransposon expression. Similarly, it was previously shown that p53–mutant females de-repress retrotransposon expression during oogenesis, but, as observed in GATOR1 mutants, primarily in the presence of meiotic DSBs. Through epistasis analysis, we determined that p53 and GATOR1 act through independent pathways to repress retrotransposon expression in the female germline. Surprisingly, we found that depletions of the TORC1 inhibitor TSC in the female germline resulted in little or no increase in retrotransposon expression. The data raise the interesting possibility that GATOR1 regulates retrotransposon expression independently of TORC1 activity. Notably, GATOR1 components, but not TSC components, were recently identified in a high-throughput screen for genes that suppress LINE1 (Long Interspersed Element-1) expression in mammalian tissue-culture cells. Taken together, our data indicate that the GATOR1 complex opposes retrotransposon expression during meiosis in a pathway that functions in parallel to p53 in the female germline of Drosophila.
The GATOR2 complex regulates the Rag GTPase–dependent recruitment of the TSC to lysosomes.
Tuberous sclerosis is a rare multiorgan genetic disorder affecting 1 in 6,000 newborns per year. Mutations in TSC components result in the hyperactivation of the metabolic regulator TORC1, causing the growth of benign tumors in many parts of the body. Although the importance of the TSC in cell metabolism is well-established, a detailed understanding of its regulation has remained elusive. We used Drosophila melanogaster and tissue culture cells to demonstrate that the GATOR2 complex is a novel regulator of TSC. The GATOR complex acts as an upstream regulator of TORC1. Its subcomplex GATOR1 inhibits the activity of TORC1 by preventing the recruitment of the complex to lysosomes where it encounters its activator Rheb. Specifically, GATOR1 regulates TORC1 localization by serving as a GTPase–activating protein for RagA/B; in their GTP–bound status, RagA/B recruit TORC1 to lysosomes.
Using fluorescence recovery after photobleaching (FRAP), we determined that knocking out WDR24, one of the subunits of the GATOR2 complex, resulted in the rapid recruitment of the TSC subunits TSC2 and TSC1 to lysosomes in both Hela cells and in the Drosophila ovary. Furthermore, we demonstrated that the GATOR2 complex regulates TSC2 dynamics by regulating the guanine nucleotide–binding status of the RagA or RagC small GTPases. Specifically, GDP–bound RagA and GTP–bound RagC promote the dynamic recruitment of TSC2 to lysosome. Moreover, by using a photoconvertible protein–tagged TSC2, we determined that the rapid association of TSC2 to lysosomes in wdr24–/– cells is accompanied by its rapid dissociation. Taking together, we provided both in vitro and in vivo evidence to support the model that the GATOR complex regulates the dynamic cycling of the TSC between lysosomes and the cytoplasm.
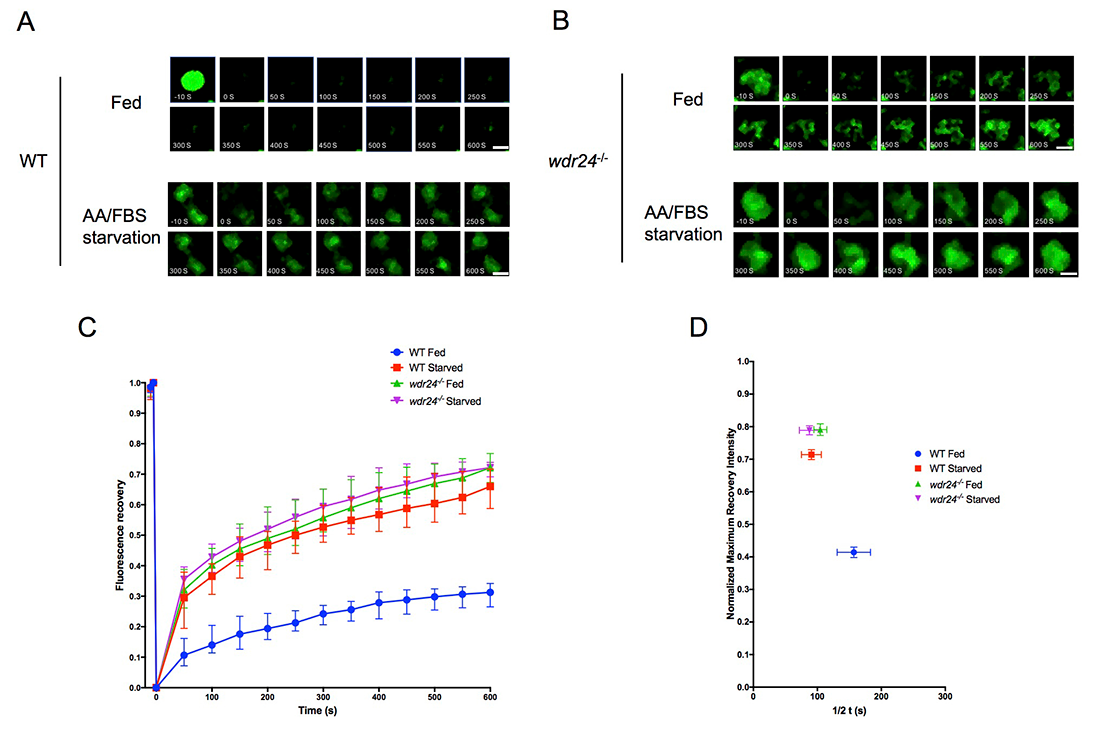
Click image to view.
Figure 3. The GATOR2 complex regulates the dynamic recruitment of TSC to lysosomes.
A. The time-point pictures in 50s intervals from after lysosomal FRAP of the Halo-TSC2 signal in WT HeLa cell. Note that fluorescent recovery was faster on lysosomes in starvation conditions than in complete medium. The 0s image represents photobleaching. Time point images were taken for 600s at intervals of 5s after photobleaching. Scale bar: 0.5 μm.
B. In WDR24-KO cell, there is no difference in the recovery of the Halo-TSC2 on lysosome in both nutrient conditions. Scale bar: 0.5 μm.
C. Fluorescence recovery versus time curves from the FRAP experiments in A and B. A total of 30 lysosomes in different cells under each treatment were analyzed. Error bar represents standard error.
D. Plot showing the relationship between the normalized maximum fluorescence recovery intensity versus half time (1/2 t) from the curves in C. Error bar represents standard error.
Publications
- Rotelli M, Policastro R, Bolling A, Killion A, Weinbeerg A, Dixon M, Zenter D, Walczak C, Lilly M, Calvi B. A Cyclin A-Myb-Aurora B network regulates the choice between mitotic cycles and polyploid endoreplication cycles. PLoS Genet 2019;15(7):e1008253.
- Wei Y, Kim K, Bettedi L, Ting CY, Zhang Y, Cai J, Lilly MA. The GATOR complex regulates an essential response to meiotic double-stranded breaks in Drosophila. eLife 2019;8:pii e42149.
- Xi J, Cai J, Cheng Y, Fu Y, Wei W, Zhang Z, Shuang Z, Hao Y, Lilly MA, Wei Y. The TORC1 inhibitor Nprl2 protects age-related digestive function in Drosophila. Aging 2019;11(21):9811-9828.
Collaborators
- Brian Calvi, PhD, Indiana University, Bloomington, IN
- R. Daniel Camerini-Otero, PhD, Genetics and Biochemistry Branch, NIDDK, Bethesda, MD
- Mary Dasso, PhD, Section on Cell Cycle Regulation, NICHD, Bethesda, MD
- Yikang Rong, PhD, San Yat-sen University, Guangzho, China
- Erik Snapp, PhD, Janelia Research Campus, Howard Hughes Medical Institute, Ashburn, VA
- Youheng Wei, PhD, Yangzhou University, China
Contact
For more information, email mlilly@helix.nih.gov or visit https://irp.nih.gov/pi/mary-lilly.


