Protein Sorting in the Endosomal-Lysosomal System

- Juan S. Bonifacino, PhD, Head, Section on Intracellular Protein Trafficking
- Rafael Mattera, PhD, Staff Scientist
- Xiaolin Zhu, RN, Technician
- Raffaella De Pace, PhD, Visiting Fellow
- Atena Farkhondeh, PhD, Visiting Fellow
- David Gershlick, PhD, Visiting Fellow
- Carlos M. Guardia, PhD, Visiting Fellow
- Rui Jia, PhD, Visiting Fellow
- Tal Keren-Kaplan, PhD, Visiting Fellow
- Sang-Yoon Park, PhD, Visiting Fellow
- Jing Pu, PhD, Visiting Fellow
- Amra Saric, PhD, Visiting Fellow
- David Kolin, BS, Postbaccalaureate Student
- Jeffrey Mercurio, BS, Postbaccalaureate Student
- Abby Moskowitz, BS, Postbaccalaureate Student
We investigate the molecular mechanisms by which transmembrane proteins (referred to as cargo) are sorted to different compartments of the endo-membrane system in eukaryotic cells. The system consists of an array of membrane-enclosed organelles including the endoplasmic reticulum (ER), the Golgi apparatus, the trans-Golgi network (TGN), endosomes, lysosomes, lysosome-related organelles (LROs) (e.g., melanosomes), and various domains of the plasma membrane in polarized cells (e.g., in epithelial cells and neurons). Transport of cargo between the compartments is mediated by carrier vesicles or tubules, which bud from a donor compartment, translocate through the cytoplasm, and eventually fuse with an acceptor compartment. Work in our laboratory focuses on the molecular machineries that mediate these processes, including (1) sorting signals and adaptor proteins that select cargo proteins for packaging into the transport carriers, (2) microtubule motors that drive movement of the transport carriers and other organelles through the cytoplasm, and (3) tethering factors that promote fusion of the transport carriers to acceptor compartments. We study the machineries in the context of different intracellular transport pathways, including endocytosis, recycling to the plasma membrane, retrograde transport from endosomes to the TGN, biogenesis of lysosomes and LROs, and polarized sorting in epithelial cells and neurons. We apply knowledge gained from this research to the elucidation of protein trafficking diseases such as the Hermansky-Pudlak syndrome (HPS), a pigmentation and bleeding disorder, and the MEDNIK syndrome, a neuro-cutaneous disorder. In addition, we study how the molecular mechanisms of protein transport are exploited by intracellular pathogens such as HIV-1.
An AP-1/clathrin pathway for the sorting of transmembrane receptors to the somato-dendritic domain of hippocampal neurons
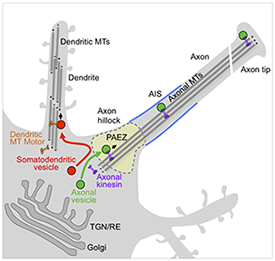
Click image to enlarge.
Figure 1. Sorting of somato-dendritic and axonal vesicles at the pre-axonal exclusion zone (PAEZ)
A major focus of research in our laboratory is on processes mediated by recognition of sorting signals in the cytosolic tails of transmembrane proteins by adaptor proteins (APs) that are components of protein coats (e.g., clathrin coats). Two types of sorting signal, referred to as tyrosine-based and dileucine-based, participate in various sorting events, including endocytosis, transport to lysosomes and melanosomes, and sorting to the basolateral surface of polarized epithelial cells. In recent years, we extended our studies to the role of signal-adaptor interactions in the process of polarized sorting in neurons. We found that many of these proteins are sorted to the somatodendritic domain by interaction of tyrosine-based or dileucine-based signals with the adaptor protein complex AP-1. More recently, in collaboration with Morten Nielsen, we found that the intracellular sorting receptor SorLA is sorted to the somatodendritic domain of neurons and the basolateral domain of polarized epithelial cells by virtue of an acidic cluster in its cytosolic tail that interacts with AP-1. Together with previous work, the findings establish the AP-1 complex as a global regulator of polarized sorting in different cell types. Defects in polarized sorting likely underlie the pathogenesis of several neuro-cutaneous disorders caused by mutation in sigma1 subunit isoforms, such as the MEDNIK syndrome (sigma1A), Fried/Pettigrew syndrome (sigma1B), and pustular psoriasis (sigma1C).
Polarized organelle segregation in neurons by differential interactions with microtubule motors
Polarized sorting of newly synthesized proteins to the somato-dendritic and axonal domains of neurons occurs by selective incorporation into distinct populations of vesicular transport carriers. We recently found that segregation of the carriers to their corresponding neuronal compartments occurs at a region in the axon hillock named the pre-axonal exclusion zone (PAEZ) through coupling to different microtubule motors. We also discovered a chain of interactors, including the small GTPase Rab5, the FHF complex, and dynein-dynactin, that retrieves somatodendritic proteins from the axon, thus contributing to their somatodendritic distribution at steady state.
A role for AP-1 in sorting presenilin-2 to late endosomes and lysosomes
In collaboration with Wim Annaert, we demonstrated an additional role for the AP-1 complex in the sorting of a novel form of gamma-secretase to late endosomes and lysosomes. Gamma-secretases are a family of intramembrane-cleaving proteases involved in the pathogenesis of Alzheimer's disease. A sorting motif in the cytosolic tail of the presenilin-2 (PSEN2) subunit of gamma-secretase targets this enzyme to late endosomes and lysosomes. The motif is recognized in a phosphorylation-dependent manner by AP-1. PSEN2 selectively cleaves late endosomal/lysosomal–localized substrates and generates a prominent pool of longer, more pathogenic amyloid-beta peptide. The findings reveal potentially important roles for lysosome-generated amyloid-beta peptide in Alzheimer’s disease.
Biochemical and functional studies of AP-4
Another adaptor protein complex named AP-4 is a component of a non-clathrin coat involved in protein sorting at the TGN. Considerable interest in this complex has arisen from the recent discovery that mutations in each of its four subunits are the cause of a congenital intellectual disability and movement disorder in humans. We discovered that an accessory protein named tepsin interacts with AP-4 via interaction of two phylogenetically conserved peptide motifs with the beta4 and epsilon ear domains of AP-4. The bivalency of the interactions increases the avidity of tepsin for AP-4 and may enable cross-linking of multiple AP-4 hetero-tetramers, thus contributing to the assembly of the AP-4 coat. The findings extend the paradigm of peptide-ear interactions, previously established for clathrin-AP-1/AP-2 coats, to a non-clathrin coat. Furthermore, in collaboration with Dennis Drayna, we identified rare sequence variants of the epsilon subunit of AP-4 in individuals with persistent developmental stuttering. We also demonstrated that AP-4 interacts with NAGPA, another protein previously linked to developmental stuttering. Our findings thus implicate deficits in intracellular trafficking in persistent stuttering.
Identification of TSSC1 as a novel component of the endosomal retrieval machinery
Endosomes function as a hub for several protein sorting events, including retrograde transport to the TGN and recycling to the plasma membrane. These processes are mediated by tubular-vesicular carriers that bud from early endosomes and fuse with a corresponding acceptor compartment. We previously investigated the role of two multi-subunit tethering complexes named GARP and EARP, which participate in SNARE (soluble N-ethylmaleimide-sensitive-factor attachment receptor)–dependent fusion of endosome-derived carriers with the TGN and recycling endosomes, respectively. More recently, we discovered that a previously uncharacterized WD40/beta-propeller protein named TSSC1 is a specific interactor of both GARP and EARP and a novel component of the endosomal retrieval machinery. Interference with TSSC1 impairs both retrograde transport to the TGN and retrieval to the plasma membrane. The findings contribute to the understanding of the pathogenesis of progressive cerebello-cerebral atrophy type 2, a neurodegenerative disorder caused by mutations in the shared Vps53 subunit of GARP and EARP.
Mechanisms of CD4 downregulation by HIV-1 Nef
Primate immunodeficiency viruses target helper T cells and macrophages/monocytes by binding of the viral envelope glycoprotein to a combination of CD4 and a chemokine receptor (CCR4 or CXCR5) on the surface of the host cells. Strikingly, infection results in rapid and sustained downregulation of CD4 and, to a lesser extent, of the chemokine receptors. Downregulation of these viral co-receptors prevents superinfection, promotes virion release and interferes with the immune response, leading to the establishment of a robust infection. CD4 downregulation is so important to the life cycle of human immunodeficiency virus-1 (HIV-1) that two accessory proteins, Nef and Vpu, encoded by the viral genome, are devoted to this task. Indeed, Nef and Vpu are critical for the progression from infection to AIDS, a fact that is best illustrated by the existence of long-term non-progressors who are infected with HIV-1 strains bearing inactivating mutations in the genes encoding these proteins. Therefore, pharmacologic or biologic perturbation of Nef and/or Vpu has the potential to prevent the pathogenic effects of HIV-1. To date, however, this potential has not been realized, mainly because Nef and Vpu have no enzymatic activity and their mechanisms of action are insufficiently understood.
In previous work, we found that the HIV-1 accessory protein Nef connects surface CD4 to both the endocytic and lysosomal targeting machineries, leading to efficient and sustained removal of CD4 from host cells early during infection. The role of Nef in CD4 internalization involves an interaction with the AP-2 clathrin adaptor. Subsequent to induction of CD4 internalization by an AP-2/clathrin pathway, Nef promotes delivery of internalized CD4 to the multivesicular body pathway (MVB) for eventual degradation in lysosomes. In collaboration with Luis da Silva, we recently found that this targeting depends on a direct interaction of Nef with Alix/AIP1, a protein associated with the Endosomal Sorting Complexes Required for Transport (ESCRT) machinery, which assists with cargo recruitment and intra-luminal vesicle formation in MVBs. We showed that Nef interacts with both the Bro1 and V domains of Alix. Depletion of Alix or overexpression of the Alix V domain impaired lysosomal degradation of CD4 induced by Nef. In contrast, V-domain overexpression did not prevent cell-surface removal of CD4 by Nef or protein targeting to the canonical, ubiquitination-dependent MVB pathway. We also showed that the Nef-Alix interaction occurs in late endosomes that are enriched in internalized CD4. Together, the results indicated that Alix functions as an adaptor for the ESCRT–dependent, ubiquitin-independent targeting of CD4 to the MVB pathway induced by Nef.
Additional Funding
- Intramural AIDS Targeted Antiviral Program (IATAP)
Publications
- Farias GG, Guardia CM, Britt DJ, Guo X, Bonifacino JS. Sorting of dendritic and axonal vesicles at the pre-axonal exclusion zone. Cell Rep 2015;10:1221-1232.
- Pu J, Schindler C, Jia R, Jarnik M, Backlund P, Bonifacino JS. BORC, a multisubunit complex that regulates lysosome positioning. Dev Cell 2015;33:176-188.
- Schindler C, Chen Y, Pu J, Guo X, Bonifacino JS. EARP is a multisubunit tethering complex involved in endocytic recycling. Nat Cell Biol 2015;17:639-650.
- Mattera R, Guardia CM, Sidhu SS, Bonifacino JS. Bivalent motif-ear interactions mediate the association of the accessory protein tepsin with the AP-4 adaptor complex. J Biol Chem 2015;290:30736-30749.
- Sannerud R, Esselens C, Ejsmont P, Mattera R, Rochin L, Tharkeshwar AK, De Baets G, De Wever V, Habets R, Baert V, Vermeire W, Michiels C, Groot AJ, Wouters R, Dillen K, Vints K, Baatsen P, Munck S, Derua R, Waelkens E, Basi GS, Mercken M, Vooijs M, Bollen M, Schymkowitz J, Rousseau F, Bonifacino JS, Van Niel G, De Strooper B, Annaert W. Restricted location of PSEN2/gamma-secretase determines substrate specificity and generates an intracellular Abeta pool. Cell 2016;166:193-208.
- Gershlick DC, Schindler C, Chen Y, Bonifacino JS. TSSC1 is novel component of the endosomal retrieval machinery. Mol Biol Cell 2016;27:2867-2878.
- Guo X, Farías GG, Mattera R, Bonifacino JS. Rab5 and its effector FHF contribute to neuronal polarity through dynein-dependent retrieval of somatodendritic proteins from the axon. Proc Natl Acad Sci USA 2016;113:E5318-5327.
Collaborators
- Wim Annaert, PhD, VIB Center for the Biology of Disease, Katholieke Universiteit, Leuven, Belgium
- Luis L. P. da Silva, PhD, Universidade de São Paulo, São Paulo, Brazil
- Dennis Drayna, PhD, Section on Genetics of Communication Disorders, NIDCD, Bethesda, MD
- Eric O. Freed, PhD, HIV Drug Resistance Program, Center for Cancer Research, NCI, Frederick, MD
- Aitor Hierro, PhD, CIC-bioGUNE, Bilbao, Spain
- Morten S. Nielsen, PhD, Aarhus Universitet, Aarhus, Denmark
- Abdul Waheed, PhD, Retroviral Replication Laboratory, Center for Cancer Research, NCI, Frederick, MD
Contact
For more information, email bonifacinoj@helix.nih.gov or visit https://irp.nih.gov/pi/juan-bonifacino.


