The Regulation or Disturbance of Protein/Lipid Interactions in Influenza, Malaria, Diabetes, Muscular Dystrophy, Brain Trauma, and Obesity

- Joshua Zimmerberg, MD, PhD, Head, Section on Integrative Biophysics
- Paul S. Blank, PhD, Staff Scientist
- Svetlana Glushakova, MD, PhD, Staff Scientist
- Vladimir A. Lizunov, MS, Research Fellow
- Petr Chlanda, PhD, Visiting Fellow
- Matthias Garten, PhD, Visiting Fellow
- Sourav Haldar, PhD, Visiting Fellow
- Brad Busse, PhD, Postdoctoral Intramural Research Training Award Fellow
- Chad McCormick, PhD, Postdoctoral Intramural Research Training Award Fellow
- Ludmila Bezrukov, MS, Chemist
- Hang Waters, MS, Biologist
- Michael J. Curran, PhD, Contractor
- Jane E. Farrington, MS, Contractor
- Elena Mekhedov, MA, Contractor
- Tatyana I. Tenkova-Heuser, PhD, Contractor
- Glen Humphrey, PhD, Guest Researcher
Eukaryotic life must create the many shapes and sizes of the system of internal membranes and organelles that inhabit the variety of cells in nature. The membranes must remodel so that cells can secrete signaling macromolecules, express surface transporters, import macromolecular cargo, store energy, repair a damaged plasmalemma, and deal with infectious agents such as viruses and parasites. Such basic membrane mechanisms must be highly regulated and highly organized in various hierarchies in space and time to allow the organism to thrive despite environmental challenges, genetic instability, an unpredictable food supply, and physical trauma. We are using our expertise and techniques that we have perfected over the years to address several different biological problems that nevertheless share the underlying regulation or disturbance of protein/lipid interactions. Our overall goal is to determine the physico-chemical mechanisms of membrane remodeling in cells and to understand the mechanisms of cellular secretion and endocytosis at physical, biophysical, and chemical levels, including the concentration and diffusion of key vesicular components prior to and after fusion or fission.
This past year, we focused on four topics: (1) elucidation of the intermediates of membrane fusion using Volta phase plate cryoelectron tomography, and the effects of cholesterol on these intermediates; (2) elucidation of the cell type that responds to the fundamental forces that emerge upon blast-like stimuli; (3) development of a new tool (resin-embedded multicycle imaging, REMI) for determining co-localized proteins using cycles of immune-staining, imaging, and stripping of the antibodies; and (4) determination of the calcium content of the endoplasmic reticulum, the first compartment that secreted proteins occupy in the secretory pathway.
Cryo-electron microscope tomography using a novel phase plate reveals intermediates of the influenza infection
The virus that causes influenza is coated with spike proteins known as hemagglutinin (HA), which play a major role in determining its immunogenicity and thus the vaccine to influenza. The HA protein plays a crucial role during infection after viral endocytosis, undergoing a conformational change that drives membrane fusion of the viral and endosomal membranes at the low pH of the endosome. Although membrane fusion is widely thought to proceed through an intermediate called hemifusion, the hemifusion structure had never been determined. In this project, we studied influenza virus–like particles carrying wild-type HA or an HA hemifusion mutant (G1S) and liposome mixtures at low pH using cryo-electron tomography. For the first time in virology, the Volta phase plate was used, a phase plate that improves the signal-to-noise ratio close to focus. We determined two distinct hemifusion structures: a hemifusion diaphragm and a novel structure termed a lipidic junction. Liposomes with lipidic junctions were ruptured with membrane edges stabilized by HA. The rupture frequency and hemifusion diaphragm diameter were not affected by the G1S mutation, but declined when the cholesterol level in the liposomes was close to physiological concentrations. We propose that HA induces a merger between the viral and target membranes by one of two independent pathways: a rupture-insertion pathway leading to the lipidic junction or a hemifusion-stalk pathway leading to a fusion pore. The latter is relevant under the conditions of influenza virus infection of cells. The cholesterol concentration functions as a pathway switch because of cholesterol's negative spontaneous curvature in the target bilayer, as determined by continuum analysis.
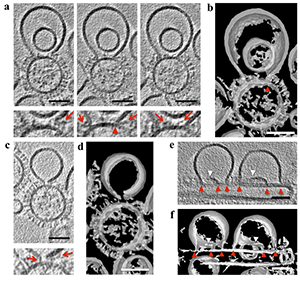
Click image to enlarge.
HA spikes in close proximity to lipidic junctions and ruptured membranes.
(a,c) Tomogram slices (3 nm thick), calculated by the weighted back projection method, from a tilt series acquired at a defocus of –1 μm with the Volta phase plate (VPP) and after nonlinear anisotropic diffusion (NAD) and Gaussian filtering, capturing a liposome containing 16 mol% cholesterol in proximity to G1S hemifusion mutant virus-like particles (VLPs) at low pH. In the left panel of (a) and in (c), the ruptured liposome membrane is in close proximity to glycoproteins. In the central and right panels of (a), the lipidic junction of the membrane sheet is inserted into the VLP membrane (red arrowhead in the magnified view below), and glycoproteins are shown inside the hemifusion diaphragm (HD) (right panel). Bottom (a,c): corresponding magnified views. Red arrows mark glycoproteins in the proximity of the HD or the open liposomal membrane. (b,d) Isosurfaces of the NAD–filtered tomographic volume calculated by the weighted simultaneous iterative reconstruction technique shown in (a) and (c), respectively, also show material within the lipidic junctions and a lack of HD completion. (e) A tomogram slice (3 nm thick), calculated by the weighted back-projection method, from a tilt series acquired at a defocus of –1 μm with VPP, showing fusion intermediates between liposomes and filamentous G1S VLPs. Red arrowheads indicate lipidic junctions, and white arrowheads indicate free membrane edges. (f) Isosurface of the NAD–filtered tomographic volume calculated by the weighted simultaneous iterative reconstruction technique shown in (e), further illustrating the lipidic junctions and free membrane edges. Scale bars, 50 nm. The tomographic slices are representative of 40 and 16 VLP–liposome interactions found in 12 and 10 tomograms collected with and without VPP on two independent grids of two independent samples, respectively.
Blast-induced traumatic brain injury results from shear forces acting on astrocytes.
Blast-induced traumatic brain injury (bTBI) continues to be a worldwide health problem. bTBI can be complex, resulting from one or more physical phases of the blast phenomenon. Even those experiencing low-level blast explosions, such as those produced by explosives used to breach fortifications, can develop neurocognitive symptoms without evidence of neurotrauma. The cellular mechanisms of the phenomenon are unknown. The primary phase of bTBI, characterized by organ-shockwave interaction, is unique to blast exposure. Understanding the mechanisms and pathology arising from the primary phase of bTBI is limited, in part because of the limited availability of in vitro models simulating the blast shockwave. It is therefore critical to develop experimental methods to study the primary phase of bTBI. To better study this phase, we developed a pneumatic device that simulates an explosive blast by producing pressure transients similar to those observed in a free-field explosion and which is compatible with real-time fluorescence microscopy of cultured cells; the device can produce blast-like pressure transients with and without accompanying shear forces. Using Ca2+ ion–selective fluorescent indicators, we could detect changes in intracellular free calcium following simulated blast. We previously showed that: (1) cultured human brain cells are indifferent to transient shockwave pressures known to cause mild bTBI; (2) when sufficient shear forces are simultaneously induced with the shockwave pressure, central nervous system (CNS) cells respond with increased intracellular Ca2+ that propagates from cell to cell; and (3) cell survival is unaffected 20 hours after shockwave exposure. We determined the cell type responsible for the waves of increased intracellular free Ca2+ in dissociated human CNS cultures, and we found that the calcium waves primarily propagate through astrocyte-dependent, purinergic signaling pathways that are blocked by P2 receptor antagonists. Human astrocytes exhibited a greater increase in calcium response than did rat astrocytes and longer calcium wave propagation kinetics, suggesting that, in our model system, rat CNS cells are less responsive to a simulated blast. Furthermore, in response to a simulated blast, expression of a reactive astrocyte marker, glial fibrillary acidic protein (GFAP), and a protease, matrix metallopeptidase 9 (MMP-9) in human CNS cells increases. The conjoint increased expression of GFAP and MMP-9 and the reduction in calcium response after application of a purinergic ATP (P2) receptor antagonist identify both potential mechanisms for sustained changes in brain function following primary bTBI and therapeutic strategies targeting abnormal astrocyte activity.
REMI: a new cell and membrane histochemical proteomic method for molecular anatomy
Protein complexes associated with cellular processes comprise a significant fraction of all biology, but our understanding of their heterogeneous organization remains inadequate, particularly for physiological densities of multiple protein species. Recent advances in microscopy allow us to localize within cells both individual proteins and some of the interactions between proteins predicted by immunoprecipitation and genetic complementation, but the sheer number of protein species involved in any one biological structure is becoming an ever more limiting factor. Many-color immuno-histochemistry, while flexible and selective, requires primary antibodies that are either directly coupled to fluorophores or from many diverse species, often forcing a shift to suboptimally affine primary antibodies. Although available immuno-histochemical labels can be supplemented with expressed markers and other methods, their potential permutations still present an overwhelming hurdle for current microscopy methods. Alternatives, such as the expression of tags for subsequent labeling, positively impact the rate at which different molecules can be imaged, but protein colocalization remains speculative with these techniques. Additionally, techniques exist to permit antibody reuse in unembedded tissue with a variety of elution methods, but the precision of subsequent labeling and tissue damage have not been documented.
Towards resolving this limitation, this year we reported a new technique based on resin-embedded multicycle imaging (REMI) of proteins in situ. By stabilizing protein structure and antigenicity in acrylic resins, affinity labels can be repeatedly applied, imaged, removed, and replaced. In principle, an arbitrarily large number of proteins of interest may be imaged on the same specimen with subsequent digital overlay. A series of novel preparative methods were developed to address the problem of imaging several protein species in areas of the plasma membrane or volumes of the cytoplasm of individual cells. For multiplexed examination of antibody staining, we used straightforward computational techniques to align sequential images and super-resolution microscopy to further define membrane protein colocalization. In one example of a fibroblast membrane with eight multiplexed proteins, a simple statistical analysis of this limited membrane proteomic dataset is sufficient to demonstrate the analytical power contributed by additional imaged proteins when studying membrane-protein domains.
Detecting intracellular calcium stores within the endoplasmic reticulum in cell models of osteogenesis imperfecta
Recessive osteogenesis imperfecta (OI) is caused by defects in proteins involved in post-translational interactions with type I collagen. Recently, a novel form of moderately severe OI caused by null mutations in TMEM38B was identified. TMEM38B encodes the endoplasmic reticulum (ER) membrane monovalent cation channel TRIC-B, which is thought to counterbalance inositol trisphosphate receptor (IP3R)–mediated Ca2+ release from intracellular stores. The molecular mechanisms by which TMEM38B mutations cause OI are unknown. We identified three probands with recessive defects in TMEM38B. TRIC-B protein is undetectable in proband fibroblasts and osteoblasts, although reduced TMEM38B transcripts are present. TRIC-B deficiency causes impaired release of ER–luminal Ca2+, associated with deficient store-operated calcium entry, although sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and IP3R have normal stability. To determine whether the lower Ca2+ flux in TRIC-B–deficient cells was associated with abnormal Ca2+ steady-state levels within the ER, we measured free ER Ca2+ using the targeted ratiometric calcium sensor D1ER. Interestingly, there was no measurable change in ER–luminal free Ca2+ in proband or normal control cells following ATP treatment, suggesting that the amount of stored Ca2+ required to elevate cytoplasmic Ca2+ is minor compared with total Ca2+ available in the ER. Furthermore, we observed no significant differences in the baseline (before ATP treatment) and depleted (after ionomycin treatment) ER Ca2+ signals between normal control and proband cells. This indicates that TRIC-B deficiency does not result in altered steady-state luminal ER free Ca2+ as, for example, a result of the abnormal Ca2+ flux. Luminal ER free Ca2+ is neither decreased nor increased.
The disturbed Ca2+ flux causes ER stress and increased BiP (binding immunoglobulin protein, involved in ER stress), and dysregulates synthesis of proband type I collagen at several steps. Collagen helical lysine hydroxylation is reduced, while telopeptide hydroxylation is increased, despite increased lysyl hydroxylase 1 (LH1) and decreased Ca2+-dependent FKBP65 (required for lysyl hydroxylase activity/access to type I collagen telopeptide lysines), respectively. Although the levels of the ER–resident protein disulfide isomerase PDI, a component of prolyl-4-hydroxylase, which is involved in collagen formation) are maintained, procollagen chain assembly is delayed in proband cells. The resulting misfolded collagen is substantially retained in TRIC-B null cells, consistent with a 50–70% reduction in secreted collagen. Lower-stability forms of collagen that elude proteasomal degradation are not incorporated into extracellular matrix, which contains only collagen with normal stability, resulting in matrix insufficiency. These data support a role for TRIC-B in intracellular Ca2+ homeostasis and demonstrate that absence of TMEM38B causes OI by dysregulation of calcium flux kinetics in the ER, impacting several collagen-specific chaperones and collagen-modifying enzymes.
Additional Funding
- Jain Foundation Award
- NICHD Director’s Award (Co-Principal Investigator with Jack Yanovski)
Publications
- Chlanda P, Mekhedov E, Waters H, Schwartz CL, Fischer ER, Ryham RJ, Cohen FS, Blank PS, Zimmerberg J. The hemifusion structure induced by influenza virus haemagglutinin is determined by physical properties of the target membranes. Nat Microbiol 2016;1:16050.
- Lizunov VA, Stenkula KG, Blank PS, Troy A, Lee J-P, Skarulis MC, Cushman SW, Zimmerberg J. Human adipose cells in vitro are either refractory or responsive to insulin, reflecting host metabolic state. PloS One 2015;10:e0119291.
- Ravin R, Blank PS, Busse B, Ravin N, Vira S, Bezrukov L, Waters H, Guerrero-Cazares H, Quinones-Hinojosa A, Lee PR, Fields RD, Bezrukov SM, Zimmerberg J. Blast shockwaves propagate Ca(2+) activity via purinergic astrocyte networks in human central nervous system cells. Sci Rep 2016;6:25713.
- Cabral WA, Ishikawa M, Garten M, Makareeva EN, Sargent BM, Weis M, Barnes AM, Webb EA, Shaw NJ, Ala-Kokko L, Lacbawan FL, Högler W, Leikin S, Blank PS, Zimmerberg J, Eyre DR, Yamada Y, Marini JC. Absence of the ER cation channel TMEM38B/TRIC-B disrupts intracellular calcium homeostasis and dysregulates collagen synthesis in recessive osteogenesis imperfecta. PLoS Genet 2016;12:e1006156.
- Busse BL, Bezrukov L, Blank PS, Zimmerberg J. Resin embedded multicycle imaging (REMI): a tool to evaluate protein domains. Sci Rep 2016;6:30284.
Collaborators
- Oleg Batishchev, PhD, A.N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russia
- Wayne A. Cabral, PhD, Bone and Extracellular Matrix Branch, NICHD, Bethesda, MD
- Fredrick S. Cohen, PhD, Rush University, Chicago, IL
- Nikki Curthoys, PhD, University of Maine, Orono, ME
- Rick M. Fairhurst, MD, PhD, Laboratory of Malaria and Vector Research, NIAID, Bethesda, MD
- Vadim Frolov, PhD, Universidad del País Vasco, Bilbao, Spain
- Hugo Guerrero-Cazares, MD, The Johns Hopkins University, Baltimore, MD
- Samuel T. Hess, PhD, University of Maine, Orono, ME
- Mary Kraft, PhD, University of Illinois at Urbana-Champaign, Urbana, IL
- Joan C. Marini, MD, PhD, Bone and Extracellular Matrix Branch, NICHD, Bethesda, MD
- Jeffery Miller, MD, Molecular Medicine Branch, NIDDK, Bethesda, MD
- Alfredo Quinones-Hinojosa, MD, The Johns Hopkins University, Baltimore, MD
- Thomas S. Reese, MD, Laboratory of Neurobiology, NINDS, Bethesda, MD
- Rolf J. Ryham, PhD, Fordham University, Bronx, NY
- Anna Shnyrova, PhD, Universidad del País Vasco, Bilbao, Spain
- Peter K. Weber, PhD, Lawrence Livermore National Laboratory, Livermore, CA
Contact
For more information, email zimmerbj@mail.nih.gov or visit irp.nih.gov/pi/joshua-zimmerberg.


