Mechanisms of Synapse Assembly, Maturation, and Growth during Development

- Mihaela Serpe, PhD, Head, Unit on Cellular Communication
- Simon Haering, PhD, Visiting Fellow
- Tae Hee Han, PhD, Visiting Fellow
- Cathy Ramos, PhD, Visiting Fellow
- Qi Wang, PhD, Visiting Fellow
- Grace Kim, BS, Postbaccalaureate Fellow
- Peter Nguyen, Biological Laboratory Technician
The purpose of our research is to understand the mechanisms of synapse development and homeostasis. The chemical synapse is the fundamental communication unit, connecting neurons in the nervous system to one another and to non-neuronal cells, and is designed to mediate rapid and efficient transmission of signals across the synaptic cleft. Such transmission forms the basis of the biological computations that underlie and enable our complex behavior. Crucial to this function is the ability of a synapse to change its properties, so that it can optimize its activity and adapt to the status of the cells engaged in communication and/or to the larger network comprising them. Consequently, synapse development is a highly orchestrated process coordinated by intercellular communication between the pre- and postsynaptic compartments and by neuronal activity itself. Our long-term goal is to elucidate the molecular mechanisms, particularly those involving cell-cell communication, that regulate formation of functional synapses during development and that fine-tune them during plasticity and homeostasis. We focus on three key processes in synaptogenesis: (1) trafficking of components to the proper site, (2) organizing those components to build synaptic structures, and (3) maturation and homeostasis of the synapse to optimize its activity. We address the molecular mechanisms underlying these processes using a comprehensive set of approaches that include genetics, biochemistry, molecular biology, super-resolution imaging, and electrophysiology recordings in live animals and reconstituted systems.
Drosophila Neto, an obligatory auxiliary subunit for neuromuscular junction (NMJ) glutamate receptors
The first step in the assembly of synapses involves recruitment of synaptic components at the proper site. Prior to the motor neuron arrival, the ionotropic glutamate receptors (iGluRs) form small, nascent clusters on the muscle, which are distributed in the vicinity of future synaptic sites. Neuron arrival at its target muscle triggers formation of large synaptic iGluRs aggregates and promotes expression of more iGluRs to permit synapse maturation and growth. The iGluR clusters interact with the local cytoskeleton and other synaptic structures to maintain local density. This involves solving two fundamental problems common to all chemical synapses: (1) trafficking the components to the proper site, and (2) organizing those components to build synaptic structures. Recent advances, particularly from vertebrate iGluR biology, reveal that the solution to these problems is completely dependent on the activity of a rich array of auxiliary subunits that associate with the receptors. These highly diverse transmembrane proteins associate with iGluRs at all stages of the receptor life-cycle and mediate the delivery of receptors to the cell surface, their distribution, synaptic recruitment, association with various postsynaptic density (PSD) scaffolds, and importantly, their channel properties. As they are assembled from different subunits, iGluRs have strikingly varied biophysical properties; their association with different auxiliary subunits increases this diversity even further. In flies as in humans, synapse strength and plasticity are determined by the interplay between different iGluR subtypes. At the fly NMJ, type-A and type-B iGluRs consist of four different subunits: either GluRIIA or -IIB, plus -IIC, -IID and -IIE. Various mechanisms regulating the extent of type-A and type-B receptor accumulation at synaptic sites have been described but the molecular mechanisms for the initial localization and clustering of receptors at synaptic sites had remained a mystery.
We recently discovered that an obligatory auxiliary protein, Neto, is absolutely required for the iGluRs clustering and NMJ functionality. To date, Neto is the only auxiliary protein characterized in Drosophila. Neto belongs to a family of highly conserved auxiliary proteins that regulate glutamatergic synapses, the major excitatory synapses in our brain. Using Neto as our entry point, we set out to elucidate the molecular mechanisms underlying the synaptic recruitment of iGluRs and their incorporation in stable neural clusters. Given that Neto does not have any catalytic activities, we hypothesized that Neto controls synapse development by interacting with iGluRs and/or with other proteins critical for synapse development. Our investigations uncovered essential roles for Neto during synapse development and strongly support the notion that trafficking of both iGluR subtypes on the muscle membrane, their synaptic recruitment and stabilization, and their function are tightly regulated by Neto. We report that Neto (a) engages in extracellular interactions that stabilize iGluRs at synaptic sites and trigger postsynaptic differentiation, (b) mediates intracellular interactions that anchor postsynaptic density components and sculpt iGluRs' postsynaptic composition, and (c) modulates iGluRs' function but not their assembly or surface delivery. More specifically, we found that Neto activities are regulated by Furin-mediated limited proteolysis, which removes an inhibitory prodomain. When the prodomain cleavage is blocked, Neto engages the iGluRs in vivo, but cannot mediate their stable incorporation/clustering at synaptic sites and fails to initiate postsynaptic differentiation. We discovered new Neto isoforms with isoform-specific roles in the recruitment of specific postsynaptic density proteins and in the selective stabilization of synaptic iGluRs. In collaboration with Mark Mayer, we reported the first functional reconstitution of Drosophila NMJ iGluRs in Xenopus oocytes and the first crystal structure for an insect iGluR, and we used the Xenopus system to examine the subunit dependence and function of these receptors. We found that, independently of Neto, four separate iGluR subunits are required for surface expression; however, Neto increases the glutamate-activated currents by several orders of magnitude. These results, which are reported in a landmark paper (Reference 5), will be key to resolving the molecular basis of the compound phenotypes observed at mutant synapses.
More recently, towards analysis of iGluRs' gating properties, we expanded our microscopy and electrophysiology toolboxes to include super-resolution imaging and fast agonist perfusion. This will allow us to parse the role of specific Neto domains and/or Neto-interacting proteins onto iGluRs' function vs. cellular trafficking. Using this methodology, we have already contributed to papers that describe new characteristics for vertebrate and insect iGluRs. A central question is how the highly conserved Neto and/or other auxiliary subunits modulate the function of highly diversified iGluRs.
A novel, noncanonical BMP pathway modulates synapse maturation at the Drosophila neuromuscular junction
Synaptic activity and synapse development are intimately linked, but our understanding of the coupling mechanisms remains limited. Anterograde and retrograde signals together with trans-synaptic complexes enable intercellular communication. How synapse activity status is monitored and relayed across the synaptic cleft remains poorly understood. The Drosophila NMJ is a powerful genetic system to study synapse development. BMP (bone morphogenetic protein) signaling modulates NMJ growth and neurotransmitter release, via a canonical pathway that is dependent on the transcription factor Smad, and synapse stability, via a noncanonical, Smad–independent pathway. Both pathways are triggered by Glass-bottom boat (Gbb), a BMP7 homolog, which binds to the presynaptic bone morphogenetic protein receptor II (BMPRII) Wishful thinking (Wit) and to the BMPRIs Thickveins (Tkv) and Saxophone (Sax). The canonical pathway activates presynaptic transcriptional programs with distinct roles in the structural and functional development of the NMJ. In the noncanonical pathway, Wit/BMPRII signal through LIM domain kinase 1 (LIMK1) to regulate actin dynamics and thus synapse stability.
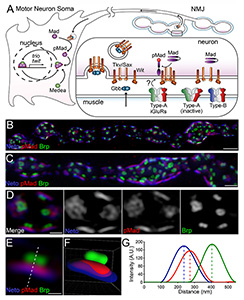
We recently discovered that phosphorylated Smad (pMad), the BMP pathway effector, accumulates at synaptic terminals in response to specific glutamate receptor subtypes, the type-A receptors. pMad accumulates at synaptic terminals at the onset of synaptogenesis and mirrors postsynaptic glutamate receptor GluRIIA throughout the NMJ development. Moreover, synaptic pMad follows the activity but not the net levels of synaptic type-A receptors, suggesting that pMad functions as a sensor for synapse activity. In collaboration with Jennifer Lippincott-Schwartz's laboratory, we used 3D structured illumination microscopy (3D-SIM) to demonstrate that pMad accumulates at the active zone (Figure 1). Our data show that synaptic pMad is distributed into discs of irregular shapes, in the close proximity to the presynaptic membrane. At Drosophila NMJ synapses, the sites of neurotransmitter release are marked by presynaptic specializations called T-bars, where Bruchpilot (Brp), the fly homolog of the vertebrate active-zone protein ELKS, accumulates. Opposite to the T-bars, the postsynaptic densities (PSDs) comprise a myriad of proteins, including Neto, that concentrate and stabilize the iGluRs. The pMad–positive signals localize between the Brp–marked T-bars and the Neto–labeled PSDs, closer to Neto.
Click image to enlarge.
Figure 1. Synaptic pMad localizes at the active zone.
(A) Diagram of BMP signaling complexes that control the accumulation of nuclear and synaptic pMad. Extracellular BMPs bind to a complex composed of Type I and Type II BMP receptors (BMPRs). The BMP/BMPR complexes are endocytosed and transported to the neuron soma, where they phosphorylate Mad and allow for of pMad's translocation to and accumulation in the motor neuron nuclei. Synaptic pMad mirrors the active postsynaptic GluRIIA and likely reflects local accumulation of BMP/BMPR complexes at synaptic terminals. (B-D) 3D-SIM images of NMJ12 boutons from third instar larvae labeled for Brp (green), pMad (red), and Neto (blue). SIM z stack maximum projections are shown in panels A and B, and a single z plane is shown in panel C. (E) High magnification view of a single synapse profile (from panel C). The line indicates the position used for the linescan plotted in panel F. (F) Side view of a surface-rendered volume of the synapse shown in panel D. (G) Intensity profile of Neto, pMad, and Brp signal along the line drawn in panel E. Linescans such as this were performed across many synapses to measure the distance of pMad and Neto from Brp.
Using genetic epistasis, histology, and electrophysiology approaches, we found that synaptic pMad is generated in the presynaptic compartment (the motor neuron) via a novel BMP signaling pathway that is genetically distinguishable from both the canonical BMP signaling and the Wit/LIMK1 noncanonical pathway. The novel pathway does not require Gbb, but depends on presynaptic Wit and Sax and postsynaptic type-A receptors. Synaptic pMad is coordinated with BMP’s role in the transcriptional control of target genes by shared pathway components but plays no role in the regulation of NMJ growth. Instead, we found that selective disruption of presynaptic pMad accumulation reduces postsynaptic GluRIIA levels, revealing a positive feedback loop that appears to function to stabilize active type-A receptors at synaptic sites. The type-A receptors are the first to arrive at a nascent synapse, whereas type-B receptors mark mature synapses. Therefore, the novel BMP signaling modality appears to be involved in sculpting synapse composition and maturation.
It is important to recognize that all these BMP signaling modalities are coordinated by shared, limited components, in particular the BMP receptors, which are tightly regulated at transcriptional, translational, and post-translational levels. The canonical BMP signaling pathway requires endocytosis of the BMP/BMPR signaling complexes and their retrograde transport to the motor neuron soma, whereas the noncanonical pathways rely on BMP/BMPR complexes' functioning at synaptic terminals. Given that the pathways share limited pools of BMPRs, the motor neurons must balance the partitioning of BMPRs among different BMP signaling modalities. Consequently, BMP signaling may monitor synapse activity and coordinate it with synapse growth and maturation.
Publications
- Sulkowski M, Han TH, Ott C, Wang Q, Verhenyer EM, Brown K, Lippincott-Schwartz J, Serpe M. A novel, noncanonical BMP pathway modulates synapse maturation at the Drosophila neuromuscular junction. PLoS Genetics 2016;12:e1005810.
- Meyerson JR, Chittori S, Merk A, Rao P, Han TH, Serpe M, Mayer ML, Subramaniam S. Structural basis of kainate subtype glutamate receptor desensitization. Nature 2016;567-571.
- Kim Y-J, Igiesuorobo O, Ramos CI, Bao H, Zhang B, Serpe M. Prodomain removal enables Neto to stabilize glutamate receptors at Drosophila neuromuscular junction. PLoS Genet 2015;11:e1004988.
- Ramos CI, Igiesuorobo O, Wang Q, Serpe M. Neto-mediated intracellular interactions shape postsynaptic composition at the Drosophila neuromuscular junction. PLoS Genet 2015;11:e1005191.
- Han TH, Poorva D, Mayer ML, Serpe M. Functional reconstitution of Drosophila NMJ glutamate receptors. Proc Natl Acad Sci USA 2015;112:6182-6187.
Collaborators
- Chi-Hon Lee, MD, PhD, Section on Neuronal Connectivity, NICHD, Bethesda, MD
- Jennifer Lippincott-Schwartz, PhD, Section on Organelle Biology, NICHD, Bethesda, MD
- Mark Mayer, PhD, Laboratory of Cellular and Molecular Neurophysiology, NICHD, Bethesda, MD
- Sriram Subramaniam, PhD, Laboratory of Cell Biology, Center for Cancer Research, NCI, Bethesda, MD
Contact
For more information, email serpemih@mail.nih.gov or visit http://ucc.nichd.nih.gov.


