Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Melanija Tomić, PhD, Staff Scientist
- Marija M. Janjic, PhD, Visiting Fellow
- Milos B. Rokic, PhD, Visiting Fellow
- Xinjie Yang, MD, PhD, Visiting Fellow
- Jovana Tavcar, MD, Special Volunteer
- Rafael M. Previde, MD, Special Volunteer (PhD Student)
We investigate cellular signaling cascades, gene expression, and hormone secretion in hypothalamic and pituitary cells, with a special emphasis on the interactions between plasma-membrane electrical events and receptor-controlled pathways. Specifically, we are addressing how these neuroendocrine cells use ion channels and G protein–coupled receptors as signaling platforms to efficiently process information. To this end, we characterize both native and recombinant receptors and channels that have been cloned from neuroendocrine cells. In the past, our work has focused on the role of inositol-trisphosphate receptors in the oscillatory calcium release of pituitary cells, the mechanism of periodic activation of these channels, and the complex mode of synchronization of calcium release from intracellular stores with electrical activity of cells. We also characterized voltage-gated channels expressed in neuroendocrine cells, the cell type–specific patterns of electrical activity and channels involved, the physiological relevance of such activity, and the crosstalk between G protein–coupled receptors and ion channels. More recently, we characterized ligand-gated receptor channels expressed in pituitary cells, including the ATP–gated P2X receptor channels. Our current work focuses on age-, sex-, and tissue structure–specific signaling, transcription and secretion in the pituitary gland, the heterogeneity of secretory pituitary cells reflecting their embryonal and postnatal genesis, and cell type–specific exocytic pathways. We are also studying how the structural features of P2X receptors relate to the channels' functions and how plasma membrane receptors and the intracellular signaling milieu affect channel activity.
Electrophysiological properties, calcium signaling, and gene expression in pituitary corticotrophs
Our recent work with corticotrophs focused on three subjects: (1) the developmental pattern of pro-opiomelanocortin gene (Pomc) expression, (2) rapid glucocorticoid effects on corticotrophs function, and (3) electrophysiological characterization of spontaneous and corticotropin-releasing hormone (CRH)–stimulated cells. The qRT-PCR analysis using pre-designed TaqMan Gene Expression Assays, which is a sensitive and reliable method to study gene expression in both male and female pituitaries in vivo and in vitro, revealed that corticotrophs show comparable expression profiles of Pomc in both sexes, with the highest expression occurring during the infantile period. Collaborative experiments with Greti Aguilera, using cultured pituitary cells, hypothalamic-derived 4B cells, and AtT-20 cells, revealed that the nongenomic/membrane effects of the classical glucocorticoid receptor mediate rapid and reversible glucocorticoid feedback inhibition at the pituitary corticotrophs downstream of calcium influx. The sensitivity and kinetics of these effects is consistent with the hypothesis that pituitary glucocorticoid feedback is part of the mechanism for adrenocortical ultradian pulse generation.
In contrast to gonadotrophs, lactotrophs, and somatotrophs, the ion channels and mechanisms controlling corticotroph excitability are still not well understood. Development of transgenic mice expressing the tdimer2(12) form of Discosoma red fluorescent protein under control of the POMC gene’s regulatory elements are a useful model for studying excitability of corticotrophs and melanotrophs. During the initial stage of investigation, done in collaboration with Greti Aguilera, we established a protocol to distinguish between these two cell types. Next, we characterized the resting membrane potential, spontaneous electrical activity, and calcium signaling in corticotrophs. The cells were either quiescent or electrically active, with a 22-mV difference. In quiescent cells, CRH depolarized the membrane, leading to initial single spiking and sustained bursting; in spontaneously firing cells, CRH further facilitated or inhibited electrical activity and calcium spiking, depending on the initial activity pattern and CRH concentration. The stimulatory action of CRH was mimicked by the cAMP activator forskolin and a cell-permeable cAMP analog. Removal of bath sodium silenced spiking and hyperpolarized the majority of cells (Figure 1); in contrast, the removal of bath calcium did not affect resting membrane potential (RMP) but reduced CRH–induced depolarization, which abolished bursting electrical activity and reduced the spiking frequency but not the amplitude of single spikes. Corticotrophs with inhibited voltage-gated sodium channels fired calcium-dependent action potentials, whereas cells with inhibited L-type calcium channels fired sodium-dependent spikes; blockade of both channels abolished spiking without affecting the RMP. These results indicate that the background voltage-insensitive sodium conductance influences resting membrane potential, the CRH–depolarization current is driven by a cationic conductance, and the interplay between voltage-gated sodium and calcium channels plays a critical role in determining the status and pattern of electrical activity and calcium signaling (Reference 1).
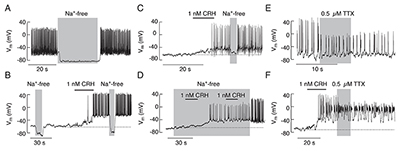
Click image to enlarge.
Figure 1. Dependence of spontaneous and CRH–induced firing of action potentials on sodium conductance in pituitary corticotrophs
A. Substitution of bath Na+ with the organic monovalent cation N-methyl-D-glucamine (NMDG+) caused deep hyperpolarization and abolished spontaneous AP firing. B and C. Removal of bath Na+ also abolished CRH–induced electrical activity. D. In cells bathed in Na+-free media, CRH was able to depolarize the plasma membrane and initiate the firing of low-amplitude APs; the amplitude and frequency of spiking increased after the return of bath Na+. E. Application of tetrodotoxin (TTX), a specific blocker of voltage-gated Na (Nav) channels, did not abolish spontaneous or (F) CRH–induced firing of APs, but reduced the amplitude of spiking.
Signature gene expression in pituitary gonadotrophs
The most obvious functional differences between mammalian males and females are related to the control of reproductive physiology and include patterns of gonadotropin-releasing hormone (GnRH) and gonadotropin release, the timing of puberty, sexual and social behavior, and the regulation of food intake and body weight. Using the rat as the best studied mammalian model for maturation, we recently examined the expression of the gonadotroph signature genes GnRH receptor gene (Gnrhr), follicle-stimulating hormone beta subunit gene (Fshb), luteinizing hormone beta subunit gene (Lhb), and alpha subunit gene (Cga) in developing males and females, using qRT-PCR and predesigned Taq-Man Gene Expression assays. Gonadotrophs exhibit highly synchronized Lhb, Fshb, Cga, and Gnrhr gene expression in both sexes, but the peak of expression occurs during the infantile period in females and at the end of the juvenile period in males. The results indicate a time shift in the peak expression during postnatal development, most likely reflecting the perinatal sex-specific brain differentiation.
In further work on this topic, we focused on basal and regulated expression of Gnrhr transcription. It is well established that hypothalamic GnRH, together with gonadal steroids and activins/inhibin, regulate the receptor's gene expression in vivo, which leads to crucial changes in GnRHR numbers on the plasma membrane. This is accompanied by alterations in gonadotroph sensitivity and responsiveness during physiologically relevant situations. However, the signaling pathways accounting for basal and regulated Gnrhr expression have not been systematically investigated. Our in vitro investigations revealed that Gnrhr expression was progressively reduced but not completely abolished in pituitary cells from adult animals cultured in the absence of GnRH and steroid hormones. The basal Gnrhr expression was also operative in LbetaT2 immortalized gonadotrophs never exposed to GnRH. In both cell types, basal transcription was sufficient for the expression of functional GnRHRs. Continuous application of GnRH transiently elevated Gnrhr expression in cultured pituitary cells followed by a sustained fall without affecting basal transcription. Both basal and regulated Gnrhr transcription was dependent on the protein kinase C signaling pathway. The GnRH–regulated Gnrhr expression was not operative in embryonal pituitary and LbetaT2 cells but was established neonatally, the sex-specific response patterns were formed at the juvenile-peripubertal stage, and there was a strong correlation between basal and regulated gene expression during development (Figure 2). Thus, age-dependent basal and regulated Gnrhr transcription could account for initial blockade and subsequent activation of the reproductive system during development (Reference 2).
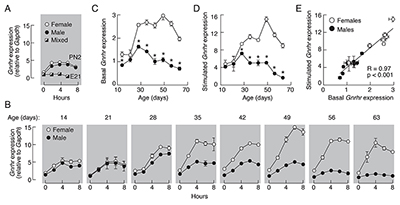
Click image to enlarge.
Figure 2. Basal and GnRH–regulated rat pituitary Gnrhr transcriptions are age and sex dependent.
A and B. Time-course studies of 10 nM GnRH–induced Gnrhr expression in embryonal pituitaries (E21), neonatal (PN2), and infant to adult pituitary cells derived from 7 to 20 animals per age group were conducted 48 h after dispersion. The age of animals in panel B is indicated on the top of gray panels. Adult female groups were composed of animals in different stages of estrous cycle. C–E. The relationship between basal and regulated Gnrhr expression in males (closed circles), females (open circles). Data are derived from panel B; the 0 time points were used for basal (C) and the peak responses were used as values for GnRH–stimulated gene expression (D). Asterisks indicate significant differences between pairs, determined by ANOVA. E. Correlation of basal and regulated rat pituitary Gnrhr expression. R, coefficient of correlation.
Dependence of P2X2R functions on its phosphorylation status and expression pattern
The purinergic P2X2 receptor (P2X2R) is an ATP–gated ion channel widely expressed in the nervous and neuroendocrine systems. Recently, we identified a putative Cdk5 (cyclin-dependent-like kinase 5, a crucial regulator of neuronal migration in the developing central nervous system) phosphorylation site in the full-size variant P2X2aR (372TPKH375), which is absent from the splice variant P2X2bR. We therefore investigated the effects of Cdk5 and its neuronal activator, p35, on P2X2aR functions. Using co-immunofluorescence and co-immunoprecipitation in HEK293 cells expressing those constructs, we found an interaction between P2X2aR and Cdk5/p35. We also found that threonine phosphorylation was significantly increased in P2X2aR. Moreover, P2X2aR–derived peptides, including the Cdk5 consensus motif, were phosphorylated in vitro by Cdk5/p35. Whole-cell patch-clamp current recordings indicated a delay in the development of use-dependent desensitization of P2X2aR but not of P2X2bR in HEK293 cells co-expressing those receptors with p35. In addition, P2X2aRs expressed in Xenopus oocytes desensitized more slowly than in HEK293 cells, and Cdk5 activation prevented this effect. The P2X2aR-T372A mutant was also resistant to use-dependent desensitization. In endogenous systems, we observed an interaction culture of nociceptive neurons. Moreover, using co-immunoprecipitation we observed interaction between Cdk5 and P2X2R in mouse trigeminal ganglia. Also, given that inhibition with roscovitine accelerated the desensitization kinetics of the currents, endogenous P2X2aR–mediated currents in PC12 cells were dependent on Cdk5 activity. The results suggest that the P2X2aR is a novel target for Cdk5–mediated phosphorylation, which might play an important role in relevant physiological processes, including pain signaling (manuscript submitted for publication).
Synaptic refinement and strengthening are activity-dependent processes, which establish orderly arranged cochleotopic maps throughout the central auditory system. The maturation of auditory brainstem circuits is guided by action potentials (APs) arising from the inner hair cells in the developing cochlea. The goal of our collaborative study with Ivan Milenkovic was to describe the tonotopic pattern of purinergic modulation during early postnatal development of the cochlear nucleus. In addition, the temporal properties of APs in neurons affected by ATP were investigated in detail using in vivo juxtacellular recordings and whole-cell recordings in acute slices. Also, we used pharmacological and molecular biological approaches to identify the P2XR subtypes accounting for effects. Using slice recordings before hearing onset and in vivo recordings with iontophoretic drug applications after hearing onset, we showed that cell-specific purinergic modulation follows a precise tonotopic pattern in the ventral cochlear nucleus of developing gerbils. In high-frequency regions, ATP responsiveness diminished before hearing onset. In low-to-mid frequency regions, ATP modulation persisted after hearing onset in a subset of low-frequency bushy cells [characteristic frequency (CF) less than 10 kHz]. Down-regulation of P2X2/3R currents along the tonotopic axis occurs simultaneously with an increase in AMPAR (glutamate receptor) currents, thus suggesting a high-to-low frequency maturation pattern. Facilitated AP generation, measured as higher firing frequency, shorter EPSP-AP (excitatory postsynaptic potential/action potential) delay in vivo, and shorter AP latency in slice experiments, is consistent with increased synaptic efficacy caused by ATP. Also, by combining electrophysiological recordings and pharmacology in vivo, in slices, and in HEK cells, we showed that the long-lasting change in intrinsic neuronal excitability is mediated by the P2X2/3R (Reference 3).
Publications
- Zemkova H, Tomic M, Kucka M, Aguilera G, Stojilkovic SS. Spontaneous and CRH-induced excitability and calcium signaling in mice corticotrophs involves sodium, calcium, and cation-conducting channels. Endocrinology 2016;157:1576-1580.
- Bjelobaba I, Janjic MM, Tavcar JS, Kucka M, Tomic M, Stojilkovic SS. The relationship between basal and regulated Gnrhr expression in rodent pituitary gonadotrophs. Mol Cell Endocrinol 2016;437:302-311.
- Jovanovic S, Radulovic T, Coddou C, Dietz B, Nerlich J, Stojilkovic SS, Rubsamen R, Milenkovic I. Tonotopic action potential tuning of maturing auditory neurons through endogenous ATP. J Physiol 2017;595(4):1315-1337.
- Trivellin G, Bjelobaba I, Daly AF, Larco DO, Palmeira L, Faucz FR, Thiry A, Leal LF, Rostomyan L, Quezado M, Schernthaner-Reiter MH, Janjic MM, Villa C, Wu TJ, Stojilkovic SS, Beckers A, Feldman B, Stratakis CA. Characterization of GRP101 transcript structure and expression patterns. J Mol Endocrin 2016;57:97-111.
- Bertram R, Tabak J, Stojilkovic SS. Ion channels and electrical activity in pituitary cells: a modeling perspective. In: MacGregor DJ, Leng G, eds. Computational Neuroendocrinology, First Edition. John Wiley & Sons. 2016;80-110.
Collaborators
- Greti Aguilera, PhD, Section on Endocrine Physiology, NICHD, Bethesda, MD
- Claudio Coddou, PhD, Faculty of Medicine, Universidad Católica del Norte, Coquimbo, Chile
- Ivan Milenkovic, PhD, Universität Leipzig, Leipzig, Germany
- Hana Zemková, PhD, Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic
Contact
For more information, email stankos@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx or irp.nih.gov/pi/stanko-stojilkovic.


