Neuregulin–ErbB Signaling in Neuronal Development and Psychiatric Disorders

- Andres Buonanno, PhD, Head, Section on Molecular Neurobiology
- Detlef Vullhorst, PhD, Staff Scientist
- Eastman Lewis, PhD, Postdoctoral Intramural Research Training Award Fellow
- Mara Bloom, BS, Postbaccalaureate Fellow
- Neha Akella, BS, Postbaccalaureate Fellow
- Hayli Spence, BS, Postbaccalaureate Fellow
- Jacqueline Welday, BS, Postbaccalaureate Fellow
- Ricardo Murphy, BS, Contractor
Failure of cortical microcircuits to properly regulate excitatory-inhibitory (E-I) balance is a key feature in the etiology of several developmental psychiatric disorders and neurological diseases, such as schizophrenia, autism, ADHD, and epilepsy. E-I balance is important to synchronize the firing pattern of local neuron ensembles, and its dysregulation can degrade cognitive functions and, in extreme cases, result in epileptiform activity. Alterations in neuronal network activity, in particular oscillations in the gamma-frequency range (30–80 Hz), are associated with behaviors and cognitive deficits in psychiatric disorders. Our Section has been investigating if and how Neuregulins (NRG 1-3) and their major neuronal receptor ErbB4, which are genetically linked to psychiatric disorders, function in an activity-dependent fashion (i.e., experience) in the developing brain to regulate synaptic and neuronal network properties. To this end, we utilized genetically modified NRG and ErbB4 mouse models, in combination with electrophysiological and molecular/cellular approaches, to identify novel interactions between the NRG/ErbB4, glutamatergic, dopaminergic, and GABAergic signaling pathways that affect E-I balance, synaptic plasticity, neuronal network activity, and behaviors repeatedly associated with cognitive deficits in psychiatric disorders.
Earlier studies focused on the functions of ErbB4 signaling in GABAergic interneurons in the hippocampus and neocortex, especially in fast-spiking parvalbumin-positive (Pv+) interneurons that modulate gamma oscillations, and in mesencephalic dopaminergic neurons. Those studies demonstrated that ErbB4 activity in GABAergic interneurons regulate gamma oscillation amplitude (power) and numerous behaviors with relevance to schizophrenia, whereas ErbB4 in mesencephalic neurons modulates extracellular dopamine levels and cognitive performance in mice. Recently, we investigated how different NRG ligands are proteolytically processed and trafficked in neurons to understand how they mediate their biological functions during brain development. NRGs are synthesized as unprocessed pro-proteins (proNRGs) that either have a single- or a dual-transmembrane (TM) domain structure. We discovered that, contrary to the dogma that all proNRGs are targeted to axons to signal in juxtracrine/paracrine fashion, single–TM proNRGs are targeted to endoplasmic reticulum–plasma membrane (ER–PM) junctions on neuronal soma, whereas dual–TM proNRGs are initially processed in the Golgi and then trafficked to axons. The differential processing and trafficking of NRGs affect their function. Single–TM NRGs are initially cleaved from somatic ER–PM junctions in response to calcium influx through NMDA receptors (NMDARs) and, in turn, the released NRGs activate ErbB4 receptors on GABAergic interneurons in autocrine fashion to promote NMDAR internalization; this novel bidirectional autocrine signaling mode can homeostatically regulate excitation onto GABAergic interneurons. By contrast, dual–TM NRGs are initially cleaved by the aspartic acid protease BACE-1 in the trans-Golgi network, sorted into axons by transcytosis, and then selectively retained at presynaptic glutamatergic terminals via juxtacrine transsynaptic interactions with ErbB4 receptors on dendrites of GABAergic interneurons, in this fashion regulating excitatory glutamatergic inputs that drive GABAergic interneurons.
Our laboratory uses a combination of experimental approaches, which include molecular/cell biology, electrophysiology, optogenetics, fiber photometry together with genetically encoded sensors, confocal fluorescence microscopy, and behavioral analyses in genetically targeted mice, with the ultimate goal of generating holistic models to investigate the developmental impact of genes that modulate the neuronal networks that underlie behaviors and cognitive functions affected in psychiatric and neurological disorders.
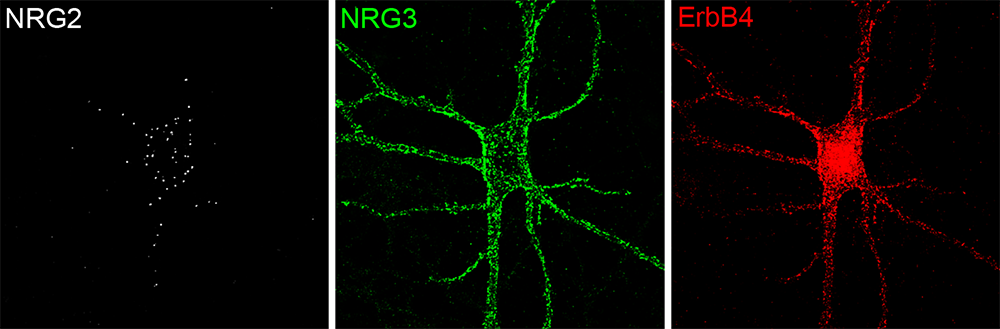
Figure 1. Distinct subcellular distribution of NRG2 and NRG3 in a cultured hippocampal ErbB4+ GABAergic interneuron
Surface puncta for pro-NRG2 (white) and processed NRG3 (green) were visualized by live labeling, followed by post-fixation labeling of ErbB4 (red). Note the restricted distribution of pro-NRG2 on the soma and proximal dendrites. In stark contrast, NRG3 and ErbB4 signals show extensive overlap, indicative of their transsynaptic interactions at excitatory synapses onto inhibitory interneurons.
Subcellular distribution, processing and function of single–TM NRGs in central neurons
We recently found that single–TM NRGs, such as NRG1 type II and NRG2, traffic as unprocessed pro-forms to the neuronal cell surface, where they accumulate at ER–PM junctions on neuronal soma and proximal dendrites (Figure 1). Shedding of the signaling-competent ectodomain of single–TM NRGs is triggered by calcium entry through excitatory glutamatergic NMDA receptors and mediated by membrane-bound ADAM (a disintegrin and metalloproteinase)–type metalloproteinases (Vullhorst et al., J Neurosci 2017;37:5232). NMDA receptor activity promotes dissociation of unprocessed proNRGs from ER–PM contact sites through dephosphorylation of multiple conserved Ser/Thr residues located in their intracellular region and ectodomain shedding by ADAM10, but not by ADAM 17 as originally proposed (Figure 2). Together, the two processes promote rapid regulated release of biologically active single–TM NRGs within minutes of NMDA receptor activation to promote ErbB4 signaling [Reference 1]. In turn, the processed NRG is released and binds to ErbB4 receptors expressed at the excitatory postsynaptic densities of GABAergic interneurons, where it selectively down-regulates NMDA, but not AMPA, receptors. We hypothesized (Vullhorst et al., Nat Comm 2015;6:7222) that this bidirectional autocrine NRG/ErbB4 signaling mode could serve as a homeostatic mechanism to regulate calcium entry in GABAergic interneurons.
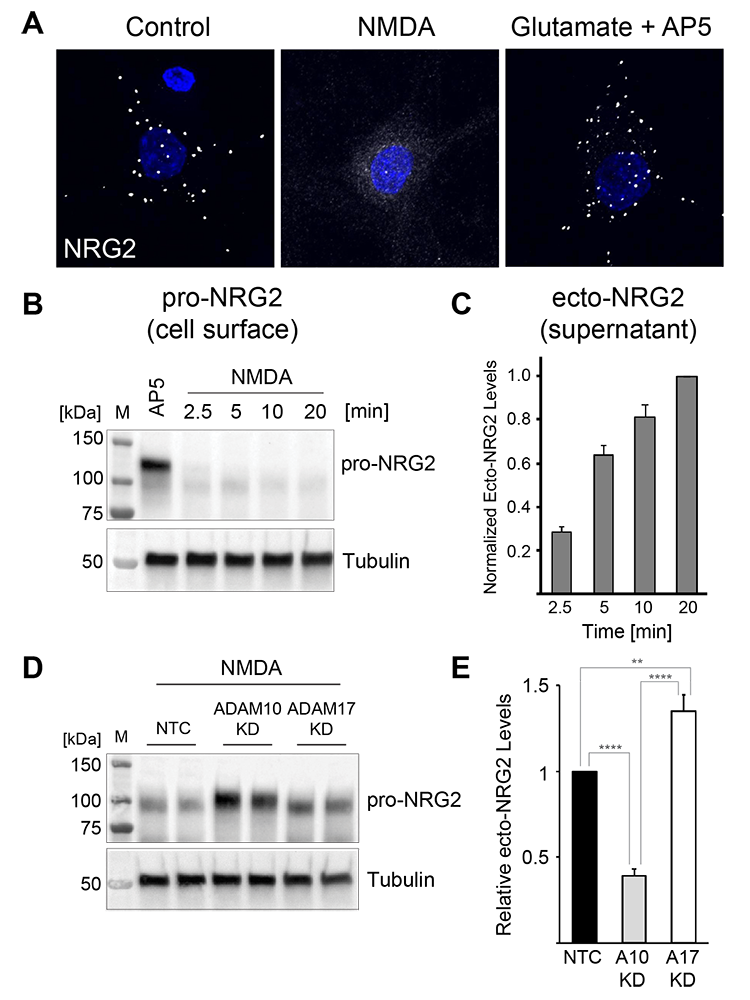
Figure 2. Activity-dependent pro-NRG2 processing by ADAM10, not ADAM17
A. Downregulation of NRG2 puncta on cultured hippocampal neurons in response to acute NMDA receptor stimulation.
B. Rapid downregulation of pro-NRG2 protein in whole-cell lysates of cultured neurons treated with 50 μm NMDA for the indicated times.
C. Accumulation of the NRG2 ectodomain in hippocampal culture supernatants following NMDA receptor stimulation, measured by ELISA.
D,E. shRNA–mediated knockdown of ADAM10, but not of the closely related ADAM17, blocks NMDAR–dependent processing of pro-NRG2 (D) and release of the NRG2 ectodomain (E).
Polarized axonal expression of dual-TM NRGs in central neurons by transsynaptic retention
In stark contrast to single–TM NRGs, dual–TM NRG1 type III and NRG3 are targeted to axons and accumulate at glutamatergic presynaptic terminals onto GABAergic interneurons, where they signal in juxtacrine mode via postsynaptic ErbB4 receptors expressed at postsynaptic densities on GABAergic interneurons (Figure 1). Our published (Vullhorst et al., Neurosci 2017;37:5232) and ongoing (Ahmad et al., submitted) studies demonstrate that cleavage of proNRG3 in the trans-Golgi network is required for its release from this organelle (Figure 3). Once cleaved near the second TM domain by BACE-1, the membrane-bound fragment containing the first cytoplasmic sequence, the TM and EGF–like domain (necessary for ErbB binding), is sorted and trafficked to axons by transcytosis. Through a novel mechanism that we denote ‘transsynaptic retention,’ the polarized accumulation of NRG3 on axonal presynaptic terminals is maintained through its transsynaptic interaction with postsynaptic ErbB4 receptors expressed on dendrites of GABAergic interneurons (Figure 3).
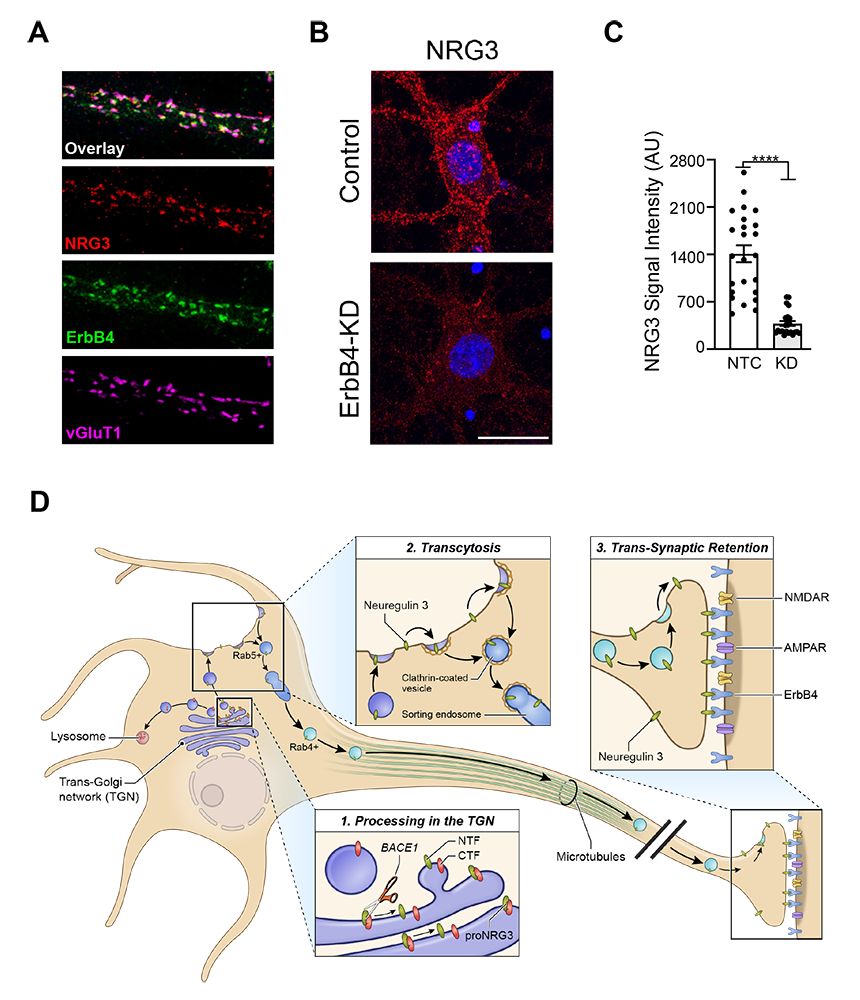
Figure 3. Stable presynaptic accumulation of mature (processed) NRG3 depends on transsynaptic retention by its cognate receptor ErbB4.
A. Immunofluorescence cytochemistry showing co-localization of NRG3, ErbB4, and the vesicular glutamate transporter 1 (vGluT1) on a cultured GABAergic interneuron dendrite.
B,C. Reduced presynaptic NRG3 signals in shRNA–treated interneurons to knock down ErbB4 expression.
D. Model summarizing the results of our NRG3 trafficking studies. Unprocessed pro-NRG3 is cleaved by the protease BACE1 in the trans-Golgi network (TGN). Processed NRG3 then traffics to the somatodendritic cell surface by transcytosis, a process that involves re-endocytosis into Rab5+ early endosomes, sorting, and anterograde axonal transport in Rab4+ vesicles. Presynaptic NRG3 is stabilized by binding of postsynaptic ErbB4 receptors.
NRG2 and ErbB4 knockout mice exhibit dopamine dysregulation and severe behavioral phenotypes with relevance to psychiatric disorders.
We found that NRG2 expression is more extensive than originally reported, extending to striatal and medial prefrontal cortical (mPFC) neurons, and its receptor ErbB4 is expressed on presynaptic process from mesencephalic dopamine (DA) neurons that innervate these structures. Therefore, to investigate the function of NRG2–ErbB4 signaling, we generated NRG2 and ErbB4 knockout (KO) mice. We found that NRG2 and ErbB4 KOs have higher extracellular DA levels in the dorsal striatum but lower levels in the mPFC and hippocampus, a pattern with similarities to DA dysbalance in schizophrenia. NRG2 and ErbB4 KO mice performed abnormally in a battery of behavioral tasks relevant to psychiatric disorders (Figure 4). They exhibit hyperactivity in a novelty-induced open field, deficits in pre-pulse inhibition, hypersensitivity to amphetamine, antisocial behaviors, reduced anxiety-like behavior in the elevated plus maze, and deficits in the T-maze alteration reward test, a task dependent on hippocampal and mPFC function. Acute administration of clozapine, a potent atypical antipsychotic, to NRG2 KO mice rapidly reduced extracellular DA levels in the mPFC and improved alternation T-maze performance (Figure 5). The work emphasizes the importance of the NRG2–ErbB4 signaling pathway on the nigrostriatal, mesocortical, and mesolimbic DA systems [References 2 and 3].
Figure 4. Overlapping behavioral and neurochemical phenotypes in NRG2 and ErbB4 KO mice
Lack of either NRG2 or ErbB4 in genetically engineered mice elicits similar phenotypic alterations with relevance to psychiatric disorders, demonstrating that NRG2 is an important and non-redundant ErbB4 receptor ligand in the postnatal brain.
| NRG2-/- | ErbB4-/- | |
|---|---|---|
| Open Field (Hyperactivity) | increased | increased |
| Elevated Plus Maze (Anxiety) | reduced | reduced |
| Prepulse Inhibition (Sensorimotor Gating) | reduced | reduced |
| T-Maze (Working Memory) | reduced | reduced |
| Amphetamine Sensitivity | increased | increased |
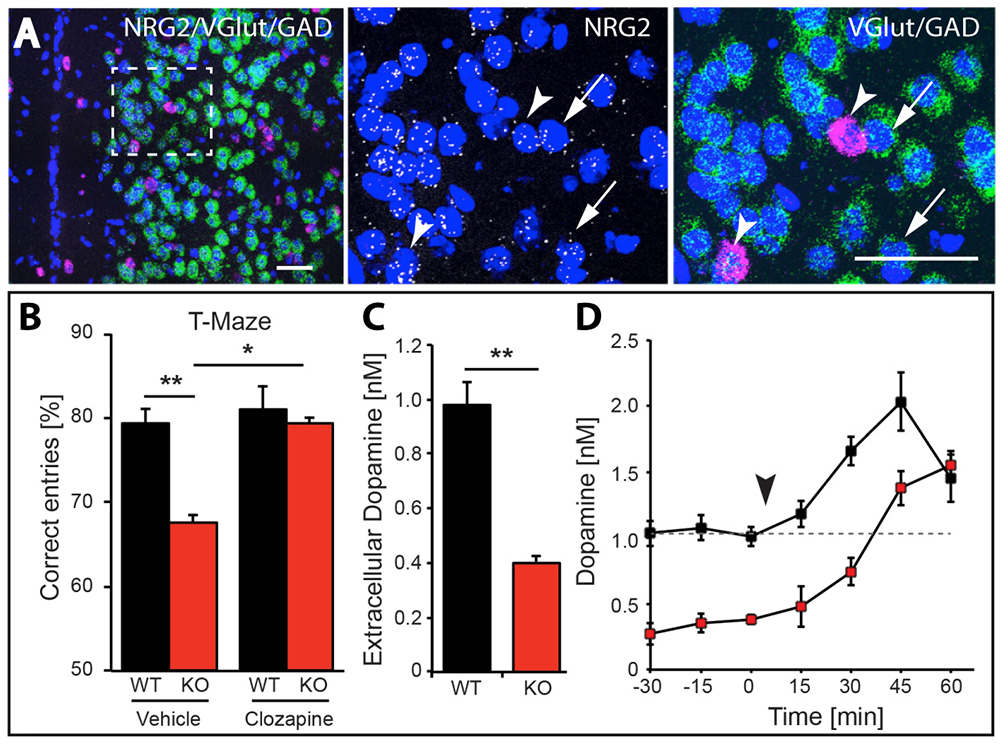
Figure 5. Working memory deficits and reduced dopamine levels in KO mice are temporarily restored by clozapine administration.
A. Expression of NRG2 in the prefrontal cortex (PFC) analyzed by triple in situ hybridization. NRG2 transcripts (white) are expressed in both glutamatergic (green) and GABAergic (magenta) neurons.
B. Poor performance by NRG2 knockout (KO) mice in a T-maze reward-alternation task, as compared with wild-type (WT) littermates (left), can be restored by administration of the antipsychotic drug clozapine (right).
C. Reduced extracellular dopamine levels in the mPFC of NRG2 KO mice.
D. Extracellular dopamine levels in the mPFC of NRG2 KO mice rise after clozapine injection (arrowhead) at a time that coincides with improved performance on the T-maze.
Analysis of ErbB4 function in mice harboring targeted mutations in either GABAergic or DAergic neurons
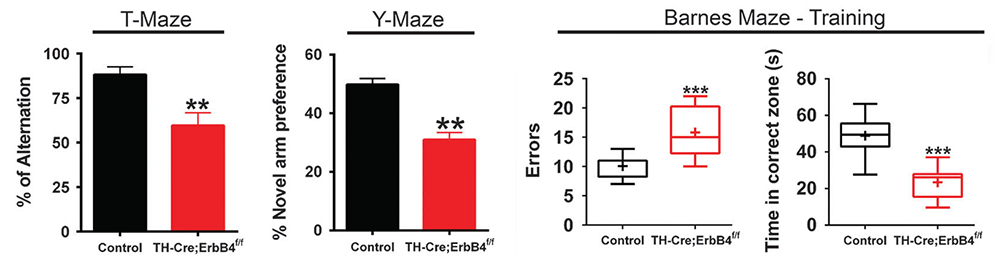
Dysfunctional NRG-ErbB4 signaling in the hippocampus, pre-frontal cortex (PFC), and striatum may contribute to alterations in DA function associated with several schizophrenia symptoms. Because NRG1 acutely increases extracellular DA levels and regulates long-term potentiation (LTP) and gamma oscillations, and ErbB4 is expressed in GABAergic (Pv+) and mesocortical DAergic (TH+) neurons, we used genetic, biochemical, and behavioral approaches to measure DA function in the hippocampus, PFC, and striatum in mice harboring targeted mutations of ErbB4 in either PV+ or TH+ neurons. Unexpectedly, we have found that, in contrast to GABAergic neurons, ErbB4 is expressed in DA neuron axons, and that NRG regulates extracellular DA levels by modulating DA transporter (DAT) function. In contrast to mice harboring mutations in GABAergic neurons, which show sensory-motor gating deficits and increases in motor activity, ErbB4 TH KO mice exhibit cognitive-related deficits in the T-, Y- and Barnes- mazes (Figure 6). Therefore, direct effects of NRG/ErbB4 signaling in GABAergic vs. DAergic neurons differentially affect cortical circuits, DA homeostasis, and behaviors relevant to schizophrenia [Reference 4].
Figure 6. Working memory deficits in mice lacking ErbB4 in DAergic neurons
Mice were assessed in three different working memory tasks (T-, Y-, and Barnes maze) and found to perform significantly less well than their control littermates. By contrast, mice lacking ErbB4 in PV+ GABAergic interneurons perform normally in these tasks (not shown).
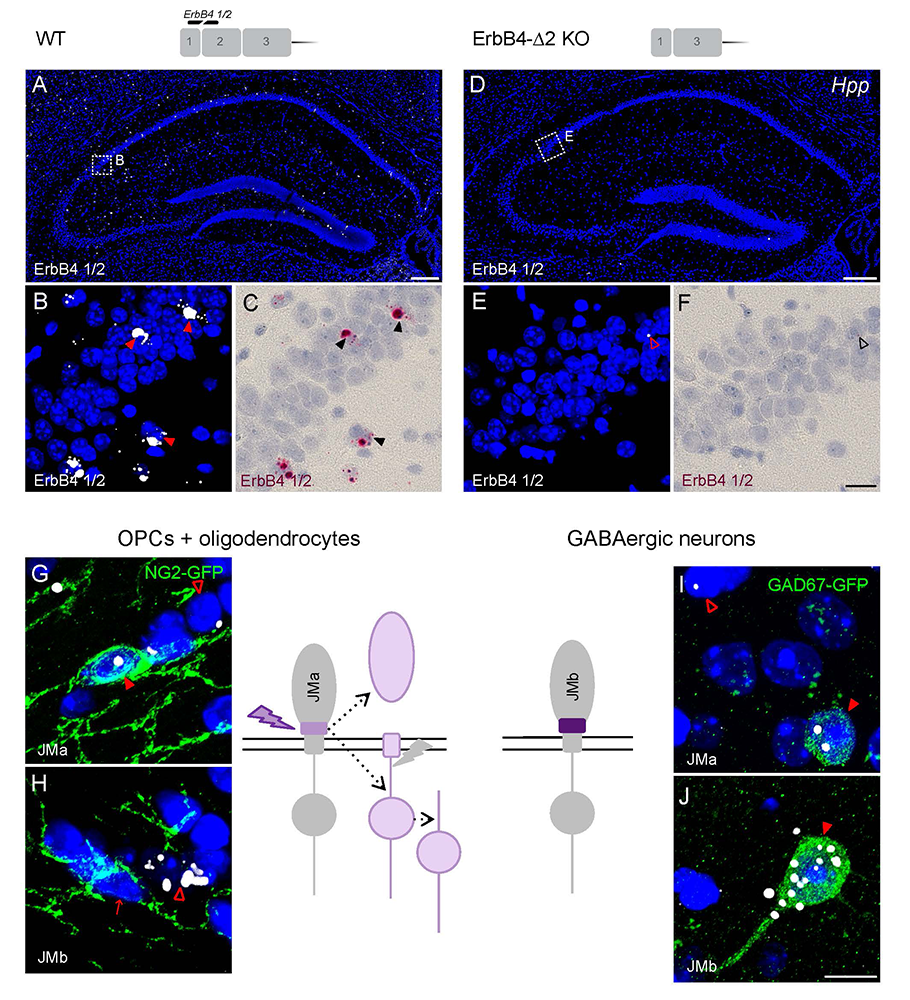
A novel ultrasensitive in situ hybridization (ISH) approach to detect short sequences and splice variants with cellular resolution
Detection of short isoform-specific sequences requires RNA isolation for PCR analysis, an approach that loses the regional and cell type–specific distribution of isoforms. The ability to distinguish the differential expression of RNA variants in tissue is critical because alterations in mRNA splicing and editing, as well as coding single-nucleotide polymorphisms, have been associated with numerous cancers and with neurological and psychiatric disorders. We reported on a novel, highly specific, and sensitive single-probe colorimetric/fluorescent ISH approach, called BaseScope, that targets short exon/exon RNA splice junctions using single-pair oligonucleotide probes. We used this approach to investigate, with single-cell resolution, the expression of four ErbB4–encoding transcripts that differ by alternative splicing of exons encoding two juxtamembrane (JMa/JMb) and two cytoplasmic (Cyt-1/Cyt-2) domains (Figure 7). First, by comparing ErbB4 hybridization on sections from wild-type and ErbB4–knockout mice (lacking exon 2), we demonstrated that single-pair probes have the specificity and sensitivity to visualize and quantify the differential expression of ErbB4 isoforms. Next, we demonstrated that expression of ErbB4 isoforms differs between neurons and oligodendrocytes. BaseScope could serve as an invaluable diagnostic tool to detect alternative spliced isoforms, and potentially single-base polymorphisms, associated with disease [References 5 and 6].
Figure 7. BaseScope analysis demonstrates that oligodendrocytes and neurons express distinct ErbB4 juxtamembrane (JM) transcripts.
The sensitivity and specificity of single-pair probe in situ hybridization was demonstrated by the presence of signals in sparse GABAergic hippocampal neurons of WT mice hybridized with a probe corresponding to exon 2 of the ErbB4 gene (A–C), and by the absence of signal in sections prepared from ErbB4 KO mice that lack exon 2 (D–F). Oligodendrocyte precursor cells (OPCs) and mature oligodendrocytes express ErbB4 JMa isoforms, which are susceptible to shedding and back-signaling (G,H), whereas GABAergic neurons express the cleavage-resistant JMb ErbB4 receptor (I,J).
Transduction of mesencephalic DAergic neurons via AAV–Cre microinjection can lead to off-target toxicity.
Stereotaxic microinjection of adeno-associated virus (AAV)–expressing Cre recombinase (AAV–Cre) into specific brain regions has become a popular approach to ablate genes by loxP/Cre recombination in a regionally and temporally specific fashion. As part of our investigations into the functional role of the ErbB4 Cyt-1 isoform in DAergic neurons, we microinjected AAV–Cre into the ventral tegmental area (VTA) of mice harboring a conditional (floxed) ErbB4 Cyt-1 allele (ErbB4 Cyt-1fl/fl mice), using AAV–Cre titers commonly reported in the literature (about 1013 genome copies [GC]/mL). To our surprise, we found that this concentration is toxic to mesencephalic DA neurons. Furthermore, we found that the toxicity is independent of the ErbB4 deletion, as AAV–Cre microinjection in both ErbB4 Cyt-1fl/fl and WT C57Bl/6J mice resulted in similar losses of DA transporter (DAT) and tyrosine hydroxylase (TH) immunoreactivity, proteins critical for DA function, and elicited behavioral changes known to be associated with loss of DA neurotransmission. Furthermore, such phenotypes were observed with different AAV serotypes and proteins expressed (Cre/GFP mixture or Cre-GFP fusion protein). Interestingly, we observed that the AAV1 serotype affects DAT and TH expression, whereas AAV9 is toxic to DAergic neurons when used at about 1013 GC/mL. Importantly, we found that diluting the viral titer 1000-fold to 1010 GC/mL effectively prevents the undesired effects while still efficiently recombining floxed targets (Erben et al., submitted). The work highlights the critical need for thorough and appropriate controls, such as including WT mice in experimental design, to account for potential off-target effects when working with AAV–Cre recombinase.
Additional Funding
- Bench-to-Bedside Award (NHD15003-001-00001) “Selective dopamine D4 receptor-targeting compounds as pro-cognitive drugs”
- NICHD DIR Director's Investigator Award (RRC# D-14-07). PI: Andres Buonanno; Co-PI: Juan Bonifacino. “Exploring the functional role of Neuregulin isoform diversity in CNS”
- Center for Compulsive Behaviors Fellowship Award
Publications
- Vullhorst D, Buonanno A. NMDA receptors regulate Neuregulin 2 binding to ER-PM junctions and ectodomain release by ADAM10. Mol Neurobiol 2019;56:8345–8363.
- Yan L, Shamir A, Skirzewski M, Leiva-Salcedo E, Kwon OB, Karavanova I, Paredes D, Malkesman O, Bailey KR, Vullhorst D, Crawley JN, Buonanno A. Neuregulin-2 ablation results in dopamine dysregulation and severe behavioral phenotypes relevant to psychiatric disorders. Mol Psychiatry 2018;23:1233–1243.
- Skirzewski M, Cronin ME, Murphy R, Fobbs W, Kravitz AV, Buonanno A. ErbB4 null mice display altered mesocorticolimbic and nigrostriatal dopamine levels as well as deficits in cognitive and motivational behaviors. eNeuro 2020;7(3):0395–19.2020.
- Skirzewski M, Karavanova I, Shamir A, Erben L, Garcia-Olivares J, Shin JH, Vullhorst D, Alvarez VA, Amara SG, Buonanno A. ErbB4 signaling in dopaminergic axonal projections increases extracellular dopamine levels and regulates spatial/working memory behaviors. Mol Psychiatry 2018;23:2227–2237.
- Erben L, He MX, Laeremans A, Park E, Buonanno A. A novel ultrasensitive in situ hybridization approach to detect short sequences and splice variants with cellular resolution. Mol Neurobiol 2018;55:6169–6181.
- Erben L, Buonanno A. Detection and quantification of multiple RNA sequences using emerging ultrasensitive fluorescent in situ hybridization techniques. Curr Protoc Neurosci 2019;87(1):e63.
Collaborators
- Tanveer Ahmad, PhD, Multidisciplinary Centre for Advance Research and Studies, Jamia Millia Islamia, New Delhi, India
- Veronica Alvarez, PhD, Section on Neuronal Structure, NIAAA, Rockville, MD
- Susan G. Amara, PhD, Laboratory of Molecular and Cellular Neurobiology, NIMH, Bethesda, MD
- Juan Bonifacino, PhD, Section on Intracellular Trafficking, NICHD, Bethesda, MD
- Steve Carroll, MD, PhD, Medical University of South Carolina, Charleston, SC
- Jung Hwa (Susan) Cheng, PhD, EM Facility, NINDS, Bethesda, MD
- Jennie Garcia-Olivares, PhD, Laboratory of Molecular and Cellular Neurobiology, NIMH, Bethesda, MD
- Carlos Guardia, PhD, Section on Intracellular Trafficking, NICHD, Bethesda, MD
- Mario A. Penzo, PhD, Unit on the Neurobiology of Affective Memory, NIMH, Bethesda, MD
- Alon Shamir, PhD, Mazor Mental Health Center, Acre, Israel
- Jung-Hoon Shin, PhD, Section on Neuronal Structure, NIAAA, Rockville, MD
- Miguel Skirzewski, PhD, University of Western Ontario, London, Canada
- Hugo Tejeda, PhD, Unit on Neuromodulation and Synaptic Integration, NIMH, Bethesda, MD
Contact
For more information, email buonanno@mail.nih.gov or visit https://smn.nichd.nih.gov.