Chemosensory Coding and Decoding by Neuron Ensembles

- Mark Stopfer, PhD, Head, Section on Sensory Coding and Neural Ensembles
- Zane Aldworth, PhD, Staff Scientist
- Alejandra Boronat Garcia, PhD, Postdoctoral Fellow
- Yu-Shan Hung, PhD, Postdoctoral Fellow
- Subhasis Ray, PhD, Postdoctoral Fellow
- Bo-Mi Song, PhD, Postdoctoral Fellow
- Kui Sun, MD, Technician
- Brian Kim, BS, Graduate Student
All animals need to know what is going on in the world around them. Brain mechanisms have thus evolved to gather and organize sensory information to build transient and sometimes enduring internal representations of the environment.
Using relatively simple animals and focusing primarily on olfaction and gustation, we combine electrophysiological, anatomical, behavioral, computational, optogenetic, and other techniques to examine the ways in which intact neural circuits, driven by sensory stimuli, process information. Our work reveals basic mechanisms by which sensory information is transformed, stabilized, and compared, as it makes its way through the nervous system.
We use three species of insects, each with specific and interlocking experimental advantages, as our experimental preparations: locusts, moths, and fruit flies. Compared with the vertebrate, the insect nervous system contains relatively few neurons, most of which are readily accessible for electrophysiological study. Essentially intact insect preparations perform robustly following surgical manipulations, and insects can be trained to provide behavioral answers to questions about their perceptions and memories. Ongoing advances in genetics permit targeting specific neurons for optogenetic or electrophysiological recording or manipulations of activity. Furthermore, the relatively small neural networks of insects are ideal for tightly constrained computational models that test and explicate fundamental circuit properties.
Response heterogeneity and adaptation in olfactory receptor neurons
The olfactory system, consisting of relatively few layers of neurons, with structures and mechanisms that appear repeatedly in widely divergent species, provides unique advantages for the analysis of information processing by neurons. Olfaction begins when odorants bind to olfactory receptor neurons, triggering them to fire patterns of action potentials. Recently, using new electrophysiological recording tools, we found that the spiking responses of olfactory receptor neurons are surprisingly diverse and include powerful and variable history dependencies. Single, lengthy odor pulses elicit patterns of excitation and inhibition that cluster into four basic types. Different response types undergo different forms of adaptation during lengthy or repeated stimuli. A computational analysis showed that such diversity of odor-elicited spiking patterns helps the olfactory system efficiently encode odor identity, concentration, novelty, and timing, particularly in realistic environments.
Feedback inhibition and its control in an insect olfactory circuit
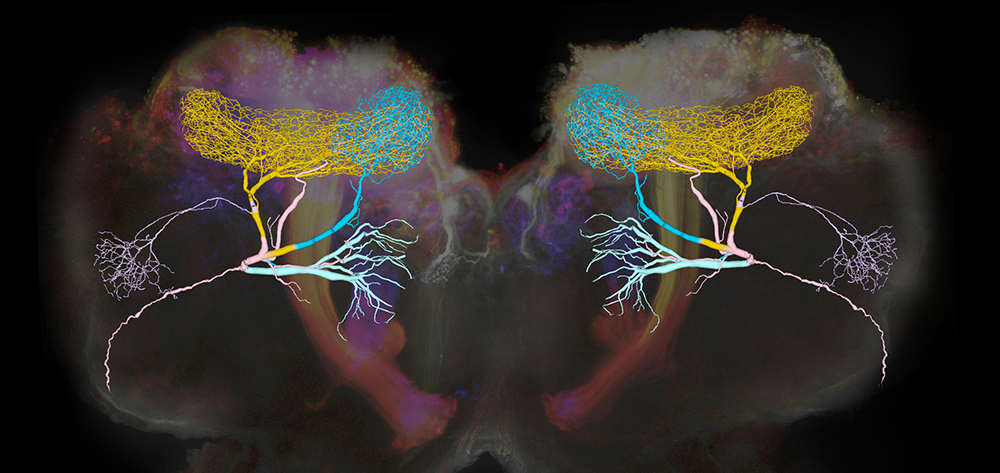
Inhibitory neurons play critical roles in regulating and shaping olfactory responses in vertebrates and invertebrates. In insects, such roles are performed by relatively few neurons, which can be interrogated efficiently, revealing fundamental principles of olfactory coding. With electrophysiological recordings from the locust and a large-scale biophysical model, we analyzed the properties and functions of the giant GABAergic neuron (GGN), a unique neuron that plays a central role in structuring olfactory codes in the locust brain (see Figure 1). Analysis of our in vivo recordings and simulations of our model of the olfactory network suggest that the GGN extends the dynamic range of Kenyon cells (high-order neurons in a brain area analogous to the vertebrate piriform cortex, which fire spikes when the animal is presented with an odor pulse), which leads us to predict the existence of a yet undiscovered olfactory pathway. Our analysis of GGN–intrinsic properties, inputs, and outputs, in vivo and in silico, reveals basic new features of this critical neuron and the olfactory network that surrounds it. Together, results of our in vivo recordings and large-scale realistic computational modeling provide a more complete understanding of how different parts of the olfactory system interact.
Figure 1. Giant GABAergic neurons regulate olfactory responses in the locust brain.
The composite image shows the structure of a compartmental computational model of the giant GABAergic neurons (GGNs) superimposed on dextran-dyed mushroom bodies in the locust brain. Different branches of GGN are shown in different colors. GGNs, only one on each side of the brain, regulate the firing of tens of thousands of olfactory neurons through feedback inhibition.
Oscillatory integration windows in neurons
Oscillatory synchronization of neurons occurs in many brain regions, including the olfactory systems of vertebrates and invertebrates, and is indispensable for precise olfactory coding. One mechanism by which oscillations have been proposed to influence coding is through the creation of cyclic integration windows, i.e., specific times within the oscillation cycle when synaptic input is most efficiently integrated by a postsynaptic neuron. Cyclic integration windows could allow a neuron to respond preferentially to spikes arriving coincidentally from several presynaptic neurons in a specific part of the cycle. Thus, coincidence detection mediated by integration windows could help read precise temporal codes for odors. Phase-specific effects of synaptic inputs have been described in both brain slices and simulations. However, the existence of cyclic integration windows has not been demonstrated, and their functional requirements are unknown.
With paired local field potential (LFP) and intracellular recordings, as well as controlled stimulus manipulations, we directly tested the idea in the locust olfactory system. We focused on the responses of Kenyon cells. We found that inputs arriving in Kenyon cells sum most effectively in a preferred window of the oscillation cycle. With a computational model, we established that the non-uniform structure of noisy activity in the membrane potential helps mediate the process. Further experiments performed in vivo demonstrated that integration windows can form in the absence of inhibition and in a broad range of oscillation frequencies.
Our results establish that cyclic integration windows can be formed from very few ingredients, i.e., oscillatory input and noise in the membrane potential. Given the ubiquity of membrane noise, the mechanisms we describe likely apply to a wide variety of neurons that receive oscillatory inputs, with or without inhibition and across a range of frequencies. Our results reveal how a fundamental coincidence-detection mechanism in a neural circuit functions to decode temporally organized spiking.
Spatiotemporal coding of individual chemicals by the gustatory system
Four of the five major sensory systems (vision, olfaction, somatosensation, and audition) are thought to be encoded by spatiotemporal patterns of neural activity. The exception is gustation. Gustatory coding by the nervous system is thought to be relatively simple, i.e., every chemical (‘tastant’) is associated with one of a small number of basic tastes, and the presence of a basic taste, rather than the specific tastant, is represented by the brain. In mammals as well as insects, five basic tastes are usually recognized: sweet, salty, sour, bitter, and umami. The neural mechanism for representing basic tastes is unclear. The most widely accepted postulate is that, in both mammals and insects, gustatory information is carried through labelled lines of cells sensitive to a single basic taste, that is, in separate channels from the periphery to sites deep in the brain. An alternative proposal is that the basic tastes are represented by populations of cells, with each cell sensitive to several basic tastes.
Testing these ideas requires determining, point-to-point, how tastes are initially represented within the population of receptor cells and how this representation is transformed as it moves to higher-order neurons. However, it has been highly challenging to deliver precisely timed tastants while recording cellular activity from directly connected cells at successive layers of the gustatory system. Using a new moth preparation, we designed a stimulus and recording system that allowed us to fully characterize the timing of tastant delivery and the dynamics of the tastant-elicited responses of gustatory receptor neurons and their monosynaptically connected second-order gustatory neurons, before, during, and after tastant delivery.
Surprisingly, we found no evidence consistent with a basic taste model of gustation. Instead, we found that the moth’s gustatory system represents individual tastant chemicals as spatiotemporal patterns of activity distributed across the population of gustatory receptor neurons. We further found that the representations are transformed substantially, given that many types of gustatory receptor neurons converge broadly upon follower neurons. The results of our physiological and behavioral experiments suggest that the gustatory system encodes information not about basic taste categories but rather about the identities of individual tastants. Furthermore, the information is carried not by labelled lines but rather by distributed, spatiotemporal activity, which is a fast and accurate code. The results provide a dramatically new view of taste processing.
Publications
- Ray S, Aldworth Z, Stopfer M. Feedback inhibition and its control in an insect olfactory circuit. eLife 2020;9:e53281.
- Alvarez-Prats A, Bjelobaba I, Aldworth Z, Takashi B, Abebe D, Kim YJ, Stojilkovic SS, Stopfer M, Balla T. Schwann-cell-specific deletion of phosphatidylinositol 4-kinase alpha causes aberrant myelination. Cell Rep 2018;23(10):2881–2890.
- Boronat-García A, Reiter S, Sun K, Stopfer M. New methods to study gustatory coding. J Vis Exp 2017;124:e55868.
- Gupta N, Singh SS, Stopfer M. Oscillatory integration windows in neurons. Nat Commun 2016;7:13808.
- Reiter S, Campillo Rodriguez C, Sun K, Stopfer M. Spatiotemporal coding of individual chemicals by the gustatory system. J Neurosci 2015;35:12309–12321.
Collaborators
- Tamás Balla, MD, PhD, Section on Molecular Signal Transduction, NICHD, Bethesda, MD
- Maxim Bazhenov, PhD, Howard Hughes Medical Institute, The Salk Institute for Biological Studies, La Jolla, CA
Contact
For more information, email stopferm@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/stopfer.