Transcriptional and Translational Regulatory Mechanisms in Nutrient Control of Gene Expression

- Alan G. Hinnebusch, PhD, Head, Section on Nutrient Control of Gene Expression
- Hongfang Qiu, PhD, Staff Scientist
- Jinsheng Dong, PhD, Senior Research Assistant
- Fan Zhang, MS, Senior Research Assistant
- Swati Gaikwad, PhD, Postdoctoral Fellow
- Michelle Gibbs, PhD, Postdoctoral Fellow
- Ritu Gupta, PhD, Postdoctoral Fellow
- Priyanka Mittal, PhD, Postdoctoral Fellow
- Poonam Poonia, PhD, Postdoctoral Fellow
- Priyanka Singh, PhD, Postdoctoral Fellow
- Vishalini Valabhoju, PhD, Postdoctoral Fellow
- Anil Vijjamarri, PhD, Postdoctoral Fellow
- Qiaoyun Zheng, PhD, Postdoctoral Fellow
We study the fundamental mechanisms involved in the assembly and function of translation initiation complexes for protein synthesis, using yeast as a model system in order to exploit its powerful combination of genetics and biochemistry. The translation initiation pathway produces an 80S ribosome bound to mRNA, with methionyl initiator tRNA (Met-tRNAi) base-paired to the AUG start codon. The Met-tRNAi is recruited to the small (40S) subunit in a ternary complex (TC) with the GTP–bound eukaryotic initiation factor eIF2 to produce the 43S preinitiation complex (PIC) in a reaction stimulated by eIFs 1, 1A, 3, and 5. The 43S PIC attaches to the 5′ end of mRNA, facilitated by the cap-binding complex eIF4F (comprising eIF4E, eIF4G, and the RNA helicase eIF4A) and poly(A)–binding protein (PABP) bound to the poly(A) tail, and scans the 5′ untranslated region (UTR) for the AUG start codon. Scanning is promoted by eIF1 and eIF1A, which induce an open conformation of the 40S and rapid TC binding in a conformation suitable for the scanning of successive triplets entering the ribosomal P site (P-out), and by eIF4F and other RNA helicases, such as Ded1 and its paralog Dbp1, that remove secondary structure in the 5′UTR. AUG recognition evokes tighter binding of the TC in the P-in state and irreversible GTP hydrolysis by eIF2, dependent on the GTPase–activating protein (GAP) eIF5, releasing eIF2-GDP from the PIC, with Met-tRNAi remaining in the P site. Joining of the 60S subunit produces the 80S initiation complex ready for protein synthesis.
Our current aims in this research area are to: (1) elucidate the functions of eIF1, eIF5, eIF3, and 40S ribosomal proteins in TC recruitment and start-codon recognition; (2) identify distinct functions of the RNA helicases eIF4A (and its cofactors eIF4G/eIF4B), Ded1, and Dbp1, and of the poly(A)–binding protein (PABP) in mRNA activation, 48S PIC assembly, and scanning in vivo; (3) uncover the mechanisms of translational repression and regulation of mRNA abundance by the repressors Scd6, Pat1, the helicase Dhh1, and the mRNA–decapping enzyme Dcp2; (4) elucidate the regulation of Ded1, eIF4G, and Dhh1 functions in response to nutrient limitation or stress; (5) elucidate the in vivo functions of yeast eIF2D orthologs and of the MCT-1/DENR complex in 40S ribosome recycling at stop codons and reinitiation in 3′ untranslated regions in vivo; and (6) elucidate the roles of yeast orthologs of eIF2A and eIF2D in eIF2–independent initiation of translation in stress conditions.
We also analyze the regulation of amino acid–biosynthetic genes in budding yeast as a means of dissecting fundamental mechanisms of transcriptional control of gene expression. During amino acid limitation, transcription of such genes is coordinately induced by the activator Gcn4 as the result of induction of Gcn4 at the translational level. The eviction of nucleosomes that occlude promoter DNA sequences and block access by RNA polymerase is thought to be a rate-limiting step for transcriptional activation. Previous studies implicated certain histone chaperones, ATP–dependent chromatin-remodeling complexes, or histone acetyltransferase (HAT) complexes in eviction of promoter nucleosomes at certain yeast genes, but it is unclear whether these co-factors function at Gcn4 target genes. Our aim is to elucidate the full set of co-factors that participate in promoter nucleosome eviction at Gcn4 target genes, their involvement in this process genome-wide, and the transcriptional consequences of defective nucleosome eviction. Functional cooperation among the chromatin-remodeling complexes SWI/SNF, RSC, and Ino80, as well as the HAT complexes SAGA, NuA4, NuA3, and Rtt109/Asf1, in these processes are under study. We recently discovered that Gcn4 can activate transcription from binding sites within the coding sequences (CDS) of its target genes, inducing internal subgenic sense and antisense (AS) transcripts in addition to the conventional full-length transcripts that initiate 5′ of the CDS; and we are probing both the mechanism and possible regulatory functions of these internal AS transcripts, as well as the roles of co-transcriptional histone methylation, nucleosome reassembly, and mRNA decay enzymes in controlling their synthesis and abundance. We are also probing mechanisms involved in the asymmetric transcriptional induction of genes belonging to pairs of divergently oriented genes where only one gene responds to Gcn4 binding at the shared upstream activation sequences (enhancer).
eIF2α interactions with mRNA control accurate start-codon selection by the translation preinitiation complex.
Comparison of previous cryo-EM structures of 48S PICs in open scanning-conducive, or closed arrested conformations revealed interactions unique to the closed complex between Arg residues R55 and R57 of domain 1 of the α-subunit of eIF2 (eIF2α-D1) with mRNA nucleotides 5′ of the AUG codon, including the –3 residue of the ‘Kozak’ context (the Kozak consensus sequence is a nucleic-acid motif that functions as the protein-translation initiation site in most eukaryotic mRNA transcripts). We showed that substitutions of R55 and R57 reduce recognition of the poor-context AUG codon for SUI1 mRNA (encoding eIF1) and also UUG start codons in Sui– cells (the Ssu– phenotype). We further showed that the R55G-R57E Ssu– substitutions destabilize TC binding to 48S PICs reconstituted with mRNA with a UUG start codon in the in vitro reconstituted system, in the manner expected from specific destabilization of the closed complex at a near-cognate codon. Interestingly, residue R53 of eIF2α-D1 interacts with rRNA residues exclusively in the open complex; we found that the R53E substitution enhances initiation at UUG codons (the Sui– phenotype) and the poor-context SUI1 AUG, and also confers the Gcd– phenotype, indicating constitutively depressed translation of GCN4 mRNA, which results from slow recruitment of the TC to scanning 40S subunits engaged in re-initiation on this mRNA in vivo. In the reconstituted system, R53E stabilized TC binding to UUG complexes while simultaneously reducing the on rate of TC loading, all in the manner predicted for specific destabilization of the open complex and shift towards the closed state. We conclude that distinct interactions of eIF2α-D1 with the rRNA or mRNA stabilize first the open, and then the closed, conformation of the PIC to regulate the accuracy and efficiency of start codon selection in vivo [Reference 1].
Large-scale movement of eIF3 domains during translation initiation modulate start-codon selection.
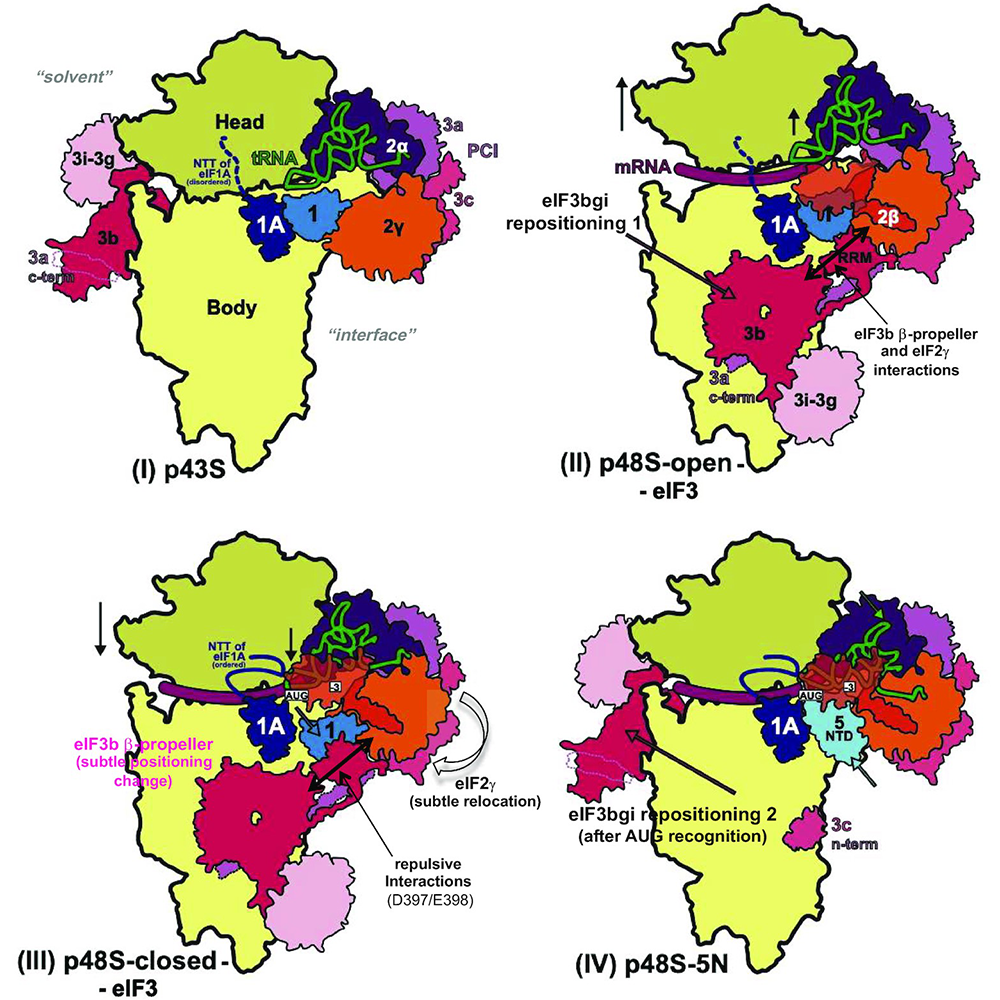
In our previous cryo-electron microscopy (cryo-EM) reconstructions of yeast 48S preinitiation complexes (PIC), the eIF3 subcomplex (dubbed the b/i/g/a module), comprising the eIF3b subunit C-terminal domain (CTD), eIF3i, the eIF3g N-terminal domain (NTD), and an extended helical segment of eIF3a-CTD, is located near the decoding center at the 40S subunit interface, interacting with eIF1, eIF2γ, eIF3c, and the 40S itself and appearing to lock the mRNA into the 40S binding cleft. The b/i/g/a module is found at this location in both the open and closed conformations of the PIC, which are thought to depict scanning and initiation conformations, respectively, with certain contacts restricted to only the open or closed state. Surprisingly, the b/i/g/a module was found at a dramatically different position on the solvent-exposed 40S surface in our more recent py48S-5N complex, where the eIF5–NTD replaces eIF1 on the 40S subunit in a later stage of the initiation pathway. We hypothesized that, following 43S PIC attachment to mRNA, the eIF3 b/i/g/a module relocates from the solvent side to the subunit interface of the open py48S complex to help prevent PIC drop-off from mRNA during scanning, that certain of its contacts at the interface surface are remodeled on AUG recognition, and that on dissociation of eIF1 and attendant loss of eIF3b–RRM (RNA recognition motif) interaction with eIF1, the b/i/g/a module relocates to the solvent side of the 40S to allow binding of the eIF5–NTD in place of eIF1 on the 40S platform. Examining eIF3b–CTD substitutions designed to disrupt interactions of its β-propeller or RRM with eIF2γ, eIF1, or eIF3c found uniquely at the interface surface revealed that those conferring the strongest phenotypes increased discrimination against near-cognate UUG start codons (the Ssu– phenotype). Binding assays confirmed the interaction of the eIF3b–RRM with eIF3c, found exclusively at the 40S subunit interface, in a manner perturbed by one such Ssu– substitution at a predicted contact with eIF3c. Interestingly, strong Ssu– phenotypes were also observed for eIF3b substitutions that perturb eIF3b interaction made exclusively at the solvent-exposed surface of the 40S subunit. The findings suggest that interactions of the b/i/g/a module with certain initiation factors at the subunit interface acts primarily to stabilize the closed conformation of the PIC on start-codon recognition, that relocation of the module back to the solvent interface is required to finalize start-codon selection, and that these interactions are crucial for the ability to utilize non-cognate initiation codons in vivo.
Figure 1. Model depicting reversible repositioning of the eIF3b/eIF3i/eIF3g/eIF3a-C-term module between the solvent-exposed and subunit-interface surfaces of the 40S at the onset of scanning and following AUG recognition
Schematics showing the two different locations observed for the eIF3b/eIF3i/eIF3g/eIF3a-C-term (eIF3 b/i/g/a) module on either the solvent or subunit surfaces of the 40S subunit in the following partial (p) initiation complexes: (I) p43S, (IV) py48S-eIF5N and (II/III) open or closed py48S complexes, respectively. The red arrows in (II) and (IV) depict the direction of the movement of the b/i/g/a module from the solvent surface to the subunit interface of the 40S subunit upon 43S PIC attachment to mRNA and formation of the open, scanning conformation of the 48S PIC (Repositioning 1), and then back to the solvent surface after AUG recognition, complete accommodation of Met-tRNAi and eIF1 dissociation from the 48S complex (Repositioning 2). Also indicated are upward or downward movements of the 40S head relative to the body (single-headed black arrows), additional interactions (two-headed black arrows) and subtle conformational changes (grey arrows or red type) of different eIF3 elements, tRNA, the 40S head and eIF2γ at the subunit interface accompanying transition from the open to closed states of the 48S complex on AUG recognition (II)-III); followed by repositioning of the eIF3b/eIF3i/eIF3g/eIF3a-Cterm module (eIF3bgi) to the solvent side of the 40S following AUG recognition, eIF1 dissociation, and replacement of eIF1 with the eIF5 NTD near the P-site (IV). Adapted from Reference 4.
Amino acid residues of 40S protein uS5/Rps2 at the mRNA entry channel enhance initiation at suboptimal start codons in vivo.
The ribosomal protein uS3/Rps3 is positioned at the solvent side of the 40S near the mRNA entry channel. We showed previously that substituting uS3/Rps3 residues that contact mRNA preferentially destabilizes the closed conformation of the PIC, reducing initiation at both UUG codons and at AUG start codons that reside in suboptimal ‘Kozak’ sequence context. Particular residues of uS5/Rps2 make distinct mRNA contacts at the 40S entry channel and also interact with rRNA elements that communicate with the 40S decoding center. We found that uS5/Rps2 substitutions V121D and I125K resemble the previously characterized uS3/Rps3 substitutions in suppressing initiation at UUG codons as well the poor-context AUG start codons in SUI1 mRNA or an elongated form of upstream open-reading frame 1 of GCN4 mRNA (el.-uORF1). Interestingly, the uS5/Rps2 substitutions D78A, Q89K, and K119A suppress UUG initiation but do not reduce initiation at the poor-context AUG codons, and thus appear to be specific for suppressing near-cognate initiation. Q94D and T96K diminish initiation at the poor-context AUG codons of SUI1 and el.-uORF1, and they efficiently suppress UUG initiation only when the UUG resides in poor sequence context. Thus, the latter two residues appear to act mainly in discriminating against poor Kozak context. The findings suggest that different uS5/Rps2 residues are involved in distinct mechanisms of discrimination against different features of poor initiation sites in vivo.
eIF4A and eIF4E interactions with distinct residues of the Ded1 N-terminus stimulate Ded1 function in translation initiation in vivo.
Binding of eIF4F to the mRNA cap structure enhances recruitment of the 43S PIC to the 5′end and subsequent scanning of the 5′UTR. Ded1 physically interacts with eIF4A and the eIF4G subunit of eIF4F, and eIF4A and eIF4G can both stimulate unwinding of a model RNA substrate by Ded1 in vitro. Previously, we showed that the Ded1 C-terminal domain (CTD) and its two interacting domains in eIF4G, dubbed RNA2 and RNA3, and the Ded1 N-terminal domain (NTD) that interacts with eIF4A, all enhance Ded1 stimulation of 48S PIC assembly in the reconstituted in vitro system. Ded1 also interacts with eIF4E; however the binding sites for eIF4A and eIF4E in the Ded1–NTD were unknown. By substituting blocks of conserved residues in the Ded1–NTD, we found that alanine replacements of residues 21–27 and 51–57 reduce Ded1 binding to eIF4A in vitro, impair association between native Ded1 and eIF4A in cell extracts, and reduce cell growth, bulk translation initiation, and translation of Ded1–hyperdependent reporter mRNAs harboring stem-loop insertions. Overexpressing eIF4A diminished the growth defects for each single substitution, but not for the 21–27/51–57 double substitution, which is null for eIF4A binding, supporting the importance of Ded1–NTD/eIF4A interaction in cells. Substituting the non-overlapping residues 59–65 and 83–89 reduced Ded1–NTD binding to eIF4E in vitro, as well as Ded1–eIF4E association in extracts, and conferred reduced translation of the Ded1–hyperdependent reporters. Combining all four NTD substitutions conferred an additive growth defect indistinguishable from deletion of the NTD, suggesting that eIF4A/eIF4E binding is the key in vivo function of the Ded1 NTD. Deleting the Ded1–CTD impairs growth only when combined with NTD substitutions, implying that the Ded1–CTD interaction with eIF4G is dispensable when Ded1 can interact with eIF4A and eIF4E. In the reconstituted system, the Ded1 NTD substitutions that eliminate eIF4A binding reduce the maximal rate of 48S PIC assembly on a Ded1–dependent mRNA harboring a 5′UTR SL (stem loop), and also increase the amount of Ded1 required to achieve the half-maximal rate (K1/2). Disruption of the Ded1–NTD/eIF4E interaction has a similar effect of elevating the Ded1 K1/2 for 48S assembly. The findings support the notion that Ded1 NTD interactions with eIF4A and eIF4E stabilize a Ded1–eIF4E–eIF4G–eIF4A quaternary complex that enhances Ded1’s ability to resolve secondary structures in 5′UTRs [Reference 2].
Reprogramming of translation in yeast cells impaired for ribosome recycling favors short, efficiently translated mRNAs.
In eukaryotes, formation of the 43S preinitiation complex (PIC), containing initiator Met-tRNAi bound to the small ribosomal subunit, is a rate-determining step of translation initiation. Ribosome recycling after translation termination produces free 40S subunits needed to reassemble 43S PICs for new initiation events. Yeast mutants lacking orthologs of mammalian eIF2D (Tma64), and either MCT-1 (Tma20) or DENR (Tma22), are broadly impaired for 40S recycling; however, it was unknown whether the defect alters the translational efficiencies (TEs) of mRNAs. Based on previous experiments, it was also possible that Tma64/eIF2D can substitute for eukaryotic initiation factor 2 (eIF2) in recruitment of Met-tRNAi during initiation. Consistent with impaired initiation, the tma64Δtma20Δ mutant exhibits reduced assembly of bulk polysomes. Ribosome profiling of this mutant reveals a marked reprogramming of translational efficiencies, wherein translation of the most efficiently translated (‘strong’) mRNAs tends to be elevated, whereas translation of ‘weak’ mRNAs generally declines. Profiling of the tma64Δ single mutant reveals none of the hallmarks of impaired 40S recycling nor changes in translation efficiencies, suggesting that the defects found in tmaΔΔ cells are associated with defective ribosome recycling rather than loss of eIF2D function in Met-tRNAi recruitment. Remarkably similar translational re-programming was seen on reducing 43S PIC assembly by inducing phosphorylation of eIF2 or by decreasing total 40S subunit levels by depleting Rps26, without affecting ribosome recycling. Moreover, the tmaΔΔ mutation specifically impaired translation of mRNAs with cap-proximal secondary structures that are expected to impede PIC attachment. Our findings suggest that strong mRNAs outcompete weak mRNAs in response to 43S PIC limitation achieved in various ways at the step of 43S PIC recruitment, in accordance with mathematical modeling of how translational efficiencies of different groups of mRNAs are altered by reduced ribosome abundance. They also have important implications for understanding changes in translation occurring in human ribosomopathies in which 40S subunit levels are diminished.
Distinct functions of three chromatin remodelers in activator binding and preinitiation complex assembly
The nucleosome-remodeling complexes (CRs) SWI/SNF, RSC, and Ino80C cooperate in evicting or repositioning nucleosomes to produce nucleosome-depleted regions (NDRs) at the promoters of many yeast genes induced by amino acid starvation. We analyzed mutants lacking the CR catalytic subunits for binding of the transcriptional activator Gcn4 and recruitment of TATA–binding protein (TBP) during preinitiation complex (PIC) assembly. RSC and Ino80 enhance Gcn4 binding to UAS (upstream activation sequence) elements in NDRs upstream of many promoters as well as to unconventional binding sites within nucleosome-occupied coding sequences; and SWI/SNF contributes to UAS binding when RSC is depleted. All three CRs are actively recruited by Gcn4 to most UAS elements and appear to enhance Gcn4 binding by reducing nucleosome occupancies at the binding motifs, indicating a positive regulatory loop. SWI/SNF acts unexpectedly in wild-type (WT) cells to prevent excessive Gcn4 binding at certain UAS elements, which might involve transient nucleosome sliding that does not alter steady-state nucleosome occupancies. All three CRs also stimulate TBP recruitment, at least partly by reducing nucleosome occupancies at TBP binding sites, with SWI/SNF acting preferentially at the most highly expressed Gcn4 target genes. RSC and Ino80 function more broadly than SWI/SNF to stimulate TBP recruitment at most constitutively expressed genes, including ribosomal protein genes, whereas SWI/SNF acts preferentially at a distinct subset of highly expressed genes. Our findings point to a complex interplay among the three CRs in evicting promoter nucleosomes to regulate activator binding and stimulate PIC assembly.
Publications
- Dong J, Hinnebusch AG. uS5/Rps2 residues at the 40S ribosome entry channel enhance initiation at suboptimal start codons in vivo. Genetics 2022;220(1):iyab176.
- Gulay S, Gupta N, Lorsch JR, Hinnebusch AG. Distinct interactions of eIF4A and eIF4E with RNA helicase Ded1 stimulate translation in vivo. eLife 2020;e58243.
- Thakur A, Gaikwad S, Vijjamarri AK, Hinnebusch AG. eIF2a interactions with mRNA control accurate start codon selection by the translation preinitiation complex. Nucleic Acids Res 2020;48:10280–10296.
- Llácer JL, Hussain T, Dong J, Villamayor L, Gordiyenko Y, Hinnebusch AG. Large-scale movement of eIF3 domains during translation initiation modulate start codon selection. Nucleic Acids Res 2021;49(20):11491–11511.
- Gaikwad S, Ghobakhlou F, Young DJ, Visweswaraiah J, Zhang H, Hinnebusch AG. Reprogramming of translation in yeast cells impaired for ribosome recycling favors short, efficiently translated mRNAs. eLife 2021;10:e64283.
Collaborators
- David Clark, PhD, Section on Chromatin and Gene Expression, NICHD, Bethesda, MD
- Chhabi Govind, PhD, Oakland University, Rochester, MI
- Jose L. Llácer, PhD, Instituto de Biomedicina de Valencia (IBV-CSIC), Valencia, Spain
- Jon Lorsch, PhD, Laboratory on the Mechanism and Regulation of Protein Synthesis, NICHD, Bethesda, MD
- Venkatraman Ramakrishnan, PhD, MRC Laboratory of Molecular Biology, Cambridge, United Kingdom
- Hussain Tanweer, PhD, Indian Institute of Science, Bangalore, India
Contact
For more information, email hinnebua@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/hinnebusch.