Translational Biophotonics in Developmental Disorders and Diseases

- Amir H. Gandjbakhche, PhD, Head, Section on Translational Biophotonics
- Hadis Dashtestani, PhD, Postdoctoral Fellow
- Helga De Oliveira Ramirez, PhD, Postdoctoral Fellow
- Kosar Khaksari, PhD, Postdoctoral Fellow
- Thien Nguyen, PhD, Postdoctoral Fellow
- Soongho Park, PhD, Postdoctoral Fellow
- Wei Lun Huang, MS, Intramural Research Training Award Student
- Emily Blick, BS, Postbaccalaureate Fellow
- Aaron Buckley, BS, Postbaccalaureate Fellow
- Sara Johnson, BS, Postbaccalaureate Fellow
- William Martin, BS, Postbaccalaureate Fellow
- John Millerhagen, BS, Postbaccalaureate Fellow
- Sheida Shahmohamadi, BS, Postbaccalaureate Fellow
- Vinay Veluvolu, BS, Postbaccalaureate Fellow
- Marc Bornstein, PhD, Special Volunteer
- Han-Shin Hahn, PhD, Special Volunteer
- Siamak Aram, PhD, Guest Researcher
- Yasaman Ardeshirpour, PhD, Guest Researcher
Brain imaging and spectroscopy of developmental disorders
We use functional near infrared spectroscopy (fNIRS) to study brain activity in two distinct lines of research: (1) developmental trajectories of cognitive abilities in children; and (2) validity of fNIRS using cognitive tasks previously evaluated using fMRI. For the developmental studies, we continued the collaboration with Audrey Thurm and Nathan Fox on the mirror neuron network (MNN) in infants. The MNN is associated with the development of sophisticated social behaviors that emerge in typical human infants (e.g., complex imitation, decoding emotional states). Modeling MNN development will create a sensitive measure of deviations in social-communication development before clinical behavioral deficits can be detected. MNN activation has already been associated with mu wave suppression using EEG. To investigate the MNN we used EEG (with high temporal resolution) in conjunction with fNIRS, which provides a more precise spatial resolution of neural activity based on hemodynamic activation. Three manuscripts were published from this work and one manuscript is under review. We conducted a review on the current fNIRS literature examining the action-observation network (AON) [Reference 1]. The review assessed and critiqued the methodological and analytic approaches that have been used to study the action-observation network in healthy adults using fNIRS. This paper was an important preparatory step in planning our empirical approach for our mirror-neuron project and contributed to the field by encouraging other researchers to consider integrating fNIRS measurement into these types of studies.
We also demonstrated the feasibility of using fNIRS to measure the neural correlates of action-observation and action-execution regions during a live task [Reference 2]. Our results indicated that the parietal regions, including bilateral superior parietal lobule (SPL), bilateral inferior parietal lobule (IPL), right supra-marginal region (SMG), and right angular gyrus (AG), share neural activity during action observation and action execution. Our findings confirm the applicability of fNIRS to the study of the AON and lay the foundation for future work with developmental and clinical populations.
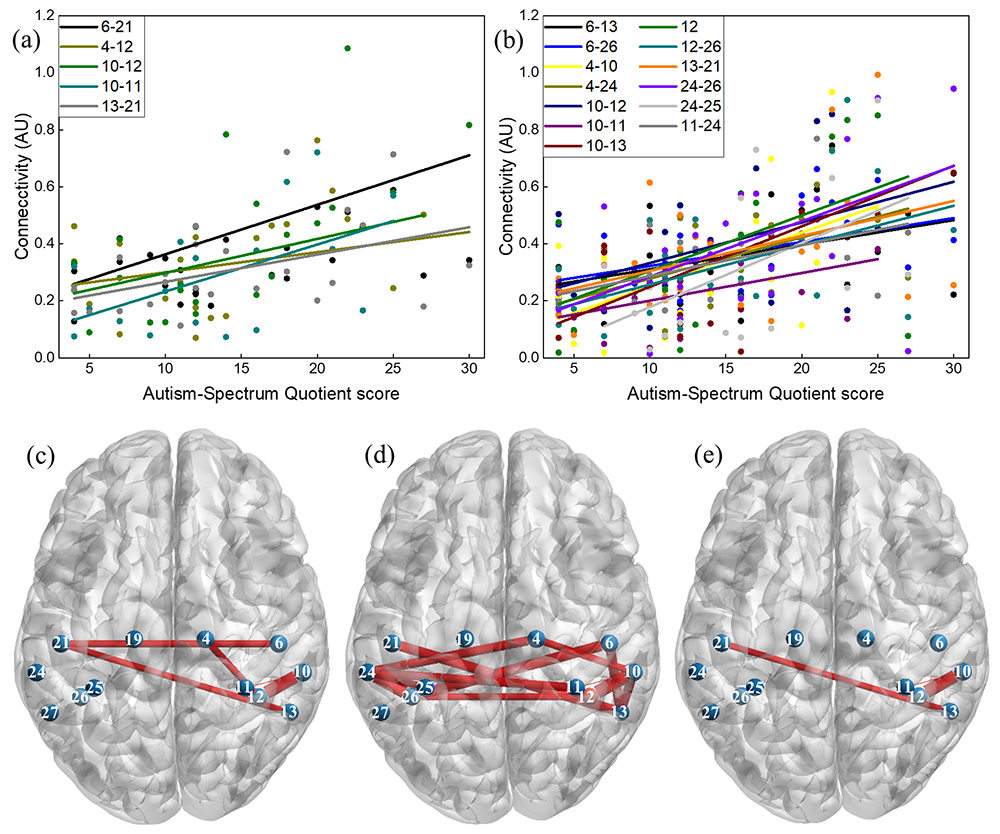
We used a functional connectivity approach to investigate functional connectivity in the MNN and assess relationship between MNN connectivity and subclinical autistic traits in neuro-typical adults [Reference 3]. In this study, we examined functional connectivity for action execution, action observation and explored MNN connectivity by identifying regions with greater connectivity in both conditions. Our results showed that during the action execution, while a participant was performing an action with the right hand, several region-to-region connections within the left hemisphere were related (connections within the left precentral, left postcentral, left inferior parietal and between the left supramarginal and left angular regions). In addition, we found five significant connections that overlapped between the two conditions: connections within the right precentral, right supramarginal, left inferior parietal, left postcentral, and between the left supramarginal and left angular regions. These connections were considered as potential candidates for mirror neuron network. Interestingly, we also found that individuals with higher subclinical autistic traits present higher connectivity in both action-execution and action-observation conditions. The results support the correlation between MNN connectivity and subclinical autistic traits can be used in future studies to investigate MNN in a developing population with autism spectrum disorder.
Figure 1. Correlations between functional connectivity and AQ score
(a) Five connections with a significant correlation coefficient during action-execution; (b) 13 connections with a significant correlation coefficient during action-observation; and brain maps showing the location of connections with significant correlation coefficients (c) during action-execution; (d) during action-observation; (e) during both conditions.
For the multimodal analysis we developed structured sparse multiset canonical correlation analysis (ssmCCA) to perform EEG-fNIRS data fusion. MCCA is a generalization of CCA to more than two sets of variables which is commonly used in medical multimodal data fusion. However, mCCA suffers from multi-collinearity, high dimensionality, unimodal feature selection, and loss of spatial information in results interpretation. A limited number of participants (small sample-size) is another problem in mCCA, leading to overfitted models. We adopted graph-guided (structured) fused LASSO (sparse) penalty to mCCA to conduct feature selection, incorporating structural information amongst variables (i.e., brain regions). Benefitting from concurrent recordings of brain hemodynamic and electrophysiological responses, the proposed ssmCCA finds linear transforms of each modality such that the correlation between their projections is maximized. Our analysis of 21 right-handed subjects indicated that the left inferior parietal region was active during both action execution and action observation. Our findings provide new insights into the neural correlates of AON which are more fine-tuned than the results from each individual EEG or fNIRS analysis and validate the use of ssmCCA to fuse EEG and fNIRS datasets. Moreover, comparing the data fusion and unimodal results, the findings from our data fusion analysis are more specific, pointing to the left inferior parietal lobe as the region that presents the highest covariation between EEG and fNIR signals during an AON paradigm. A paper related to EEG-fNIRS data fusion is currently under review. Taken together, the studies derived from our pilot study validate the MNN live paradigm to study infants at risk of neuro-developmental disorders, namely autism. We were unable to recruit infant participants in the past year owing to the COVID-19 pandemic but plan to start recruitment during the current year. Once we have tested the paradigm in a subset of typically developing infants, we will start recruiting infants at risk.
We continued our collaboration with Andrea Gropman to examine developmental deficits in children with urea cycle disorders (UCD), specifically ornithine transcarbamylase deficiency (OTCD), which is characterized by presence of hyperammonia (HA). HA is known to cause impairments in executive function and working memory. Monitoring OTCD progression and investigating neurocognitive biomarkers can become critical in examining the underlying brain function in OTCD. Using fNIRS we examined the hemodynamics of the prefrontal cortex (PFC) in an OTCD population and in fraternal twins with and without OTCD. Results revealed a distinction in left PFC activation between controls and patients with OTCD, where controls showed higher task related activation increase while performing the Stroop task (Anderson et al., Front Neurol 2020;11:809). Subjects with OTCD also exhibited a bilateral increase in PFC activation. We quantified the hemodynamic variations in total-hemoglobin, while twins performed the N-Back Working Memory task. Our preliminary results showed that the sibling with OTCD had higher variations in a very low frequency band than did the control sibling, possibly owing to the effect of HA (Anderson et al., Front Neurol 2020;11:809). Functional connectivity (FC) analysis also revealed lower interhemispheric FC in an OTCD sibling as the task load increased.
As part of our ongoing fNIRS calibration protocol, two articles were published. The first [Reference 3] reported simultaneously collected fNIRS from the PFC and high-frequency heart rate variability (HF-HRV, as derived from electrocardiogram) to examine the connection between prefrontal activation and parasympathetic nervous system activity (as measured HF-HRV) during a behavioral flexibility task (the go/no-go task). The relationship between these measures was previously described by the neuro-visceral integration model (Thayer & Lane, J Atten Disord 2000;61:201); however, so far, no study had examined such measures simultaneously. We collected data from 38 healthy adult controls at rest and during the go/no-go task. We then compared the time course of HF-HRV and prefrontal fNIRS activation over the baseline period with that of the task period to determine whether they were related over time. Results indicated that, at rest, HF-HRV is negatively related to prefrontal activation, consistent with previous studies that had collected HF-HRV and brain activity during separate resting state sessions (Allen B et al., Psychophysiology 2015;52:277). The results support the tenets of the neuro-visceral integration model and the utility of fNIRS in future studies to examine the model and its relation to cognitive functions. The second publication [Reference 5] presented a secondary data analysis on the go/no-go task used in the above study to examine prefrontal connectivity during a simple go/no-go task and an emotional go/no-go task. We found that stronger connectivity was associated with better performance on the simple task in both males and females; however, stronger connectivity was only associated with better performance on the emotional go/no-go in males. The findings are relevant to how the brain may function differently during emotional behavior inhibition in males than in females and will be further explored.
COVID-19 point-of-care biosensor
The COVID-19 pandemic is challenging the medical community to develop biosensors to detect patients with early signs of COVID-19 infection. Consequently, we developed and tested a multimodal sensing device for monitoring parameters related to physiological changes in respiratory infections. The device consists of a near-infrared spectroscopy (NIRS) device, a photoplethysmography (PPG) device, and three sensors for temperature sensing. NIRS is a non-invasive method for measuring blood hemoglobin concentrations using a near-infrared (NIR) light source. PPG is another optical method to detect changes in blood volume at the microvascular level. Respiratory function and cardiac parameters can be obtained from NIRS and PPG signals, respectively. The device's NIRS sensor differs from conventional NIRS sensors in that it uses a three-wavelength NIR source (730 nm, 810 nm, and 850 nm) with three source-detector distances of 2 cm, 3 cm, and 4 cm. The study is a collaboration with Bruce Tromberg's Section and with Babak Shadgan. We started to recruit subjects at the NIH through a clinical study. At the University of British Columbia, 20 subjects have been studied so far. Preliminary data showed significant differences in respiratory parameters detected by the device between normal, healthy breathing, and breathing that simulated what is observed in patients with a respiratory infection.
Placenta oxygenation: from basics to point of care
The placenta plays an essential role in the health of both mother and fetus. An abnormal placenta is associated with pregnancy complications such as preeclampsia, fetal growth restriction, fetal death, preterm labor, and other complications. NIRS is an optical method for non-invasive measurement of blood oxygenated and deoxygenated hemoglobin and tissue oxygenation in deep tissue layers such as brain, muscle, and placenta. However, studies examining placental oxygenation levels have yielded conflicting results, discrepancies that may be the result of unknown placental scattering coefficients used for oxygenation calculations or differences in patient populations. A major problem in the assessment of placental oxygenation using NIRS arises from the anatomical location of the organ. Taking into account the anatomical location of the maternal placenta (e.g., skin, adipose tissue, uterine wall), we designed a new wearable depth-resolving NIRS device featuring six source-detector distances in the range of 10–60 mm. Distinct source and detector distances scan different tissue depths to help distinguish between placental and maternal oxygenation. The device also uses two light sources with wavelengths of 760 nm and 840 nm so that it is sensitive to changes in blood oxyhemoglobin and deoxyhemoglobin. We evaluated the performance of the NIRS device by observing changes in the optical properties of a placental-mimicking phantom at a depth of 25 mm. In addition, to evaluating the accuracy and validating the performance of the custom NIRS device, we performed in vivo oxygen measurements on two human subjects using a wearable NIRS device in various parts of the body, including arms, calves, and abdomen. We also compared the wearable NIRS device with a time-domain NIRS system (TRS-41 system, Hamamatsu Photonics, Japan) on different parts of the body, including the arms, calves, and abdomen to evaluate the accuracy and validate the performance. We found an average error of 2.7% ± 1.8% between the two devices/systems, an agreement between the measurements of the wearable NIRS device and the well established TRS system that validates the high accuracy of the device in in vivo tissue oxygenation measurements.
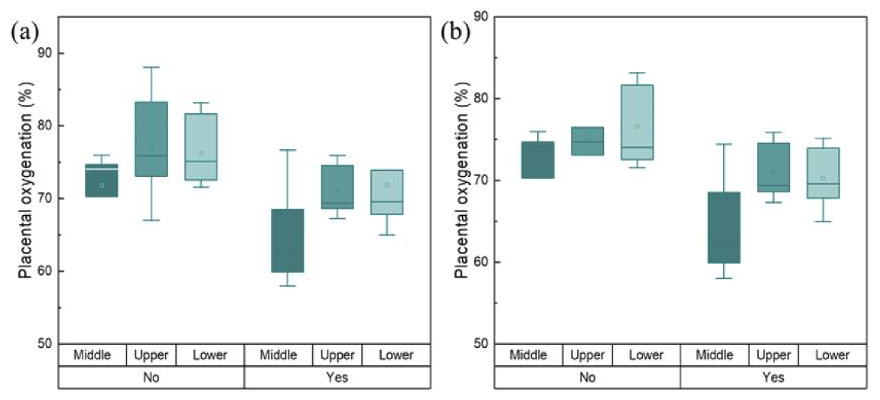
Figure 2. Transabdominal placental oxygenation levels at different measurement positions above the placenta
(a) Pregnancy with maternal complications (yes, n = 5 at each position) and no complications (no, n = 7 at each position); (b) placenta with lesions (yes, n = 5 at each position) and without lesions (no, n = 5 at each position).
We then used the NIRS device to measure placental oxygenation in vivo in 12 volunteers from the Advanced Obstetrics and Gynecology Research Center located at Detroit Medical Center (DMC). Measurements were performed at three locations: upper, middle, and lower parts of the placenta. Of the 12 subjects, five had maternal pregnancy complications such as short cervix, hypertension, or polyhydramnios. Eleven of the 12 participants delivered at the DMC. After delivery, the placenta of 10 of 11 participants was transferred to the DMC pathology department to examine the lesion. Chronic or acute lesions were found in five placentas, four of which were from participants with maternal pregnancy complications. The level of placental oxygenation was calculated using the intensity of backscattered light at the appropriate light source-detector separation. For each patient, three oxygenation levels at the three measurement sites were used: the upper, middle, and lower parts of the placenta. Our results suggest a possible relationship between placental oxygenation levels and pregnancy complications and placental pathology. However, the sample size used in this study is small (12 participants). We will conduct further studies to include more subjects. Results from this study have been published [Reference 4]. We are performing Monte Carlo simulations on the four-layer model to better understand the experimental results and to create theoretical indicators. Simulations are based on thickness and scattering and absorption coefficients of all maternal layers (dermis, adipose, uterus/rectus) and placenta. Based on Monte Carlo's random walk theory and experimental results, we are attempting to develop a system that can analyze the characteristics of light scattering and propagation in the mother's maternal layers and more clearly measure the oxygenation levels of the mother's placenta.
Also, we are developing an analysis algorithm that evaluates the behavior of placental cells in consideration of various oxygen levels using the dynamic full-field optical coherence tomography (DFFOCT) system in parallel with measurement of placental oxygenation using the NIRS device. We are developing an algorithm designed to analyze changes in the dynamic activity (frequency and magnitude of cells) within a cell and calculate a mean frequency representing a weighted frequency. As a preliminary experiment, we evaluated the viability status of HeLa cells, an immortal human cell line widely used in cell research, from alive to dead. With the developed algorithm, the dynamic activity of cells was quantitatively and clearly distinguishable according to changes in their viability status [Reference 6]. From these results, we believe that DFFOCT can be used to analyze changes in cellular dynamic activity depending on the nutrient and oxygen saturation contained in placental cells. We hypothesize that there is a relationship between the dynamic activity of placental cells and potential neuro-developmental disorders. For the next study, we will analyze the dynamic activity of the placenta cells, taking into account various oxygen levels in the placenta. To this end, we plan to create a special sample chamber to control and maintain the oxygen concentration in the cells while conducting the experiment.
Figure 3. Dynamic and structural changes in HeLa cells over time.
The frequency component with the dominant dynamic motion of the scatterers is prominently displayed in the image. The intracellular migration of main dynamic active elements is indicated by white arrows. The power density was normalized.
Additional Funding
- Bench to Bedside Award 345 (2016): “Mirror neuron network dysfunction as an early biomarker of neurodevelopment” (ongoing)
- Human Placenta Project—NICHD (2016, ongoing)
Publications
- Condy EE, Miguel HO, Millerhagen J, Harrison D, Khaksari K, Fox N, Gandjbakhche A. Characterizing the action-observation network through functional near-infrared spectroscopy: a review. Front Hum Neurosci 2021;15:627983.
- Miguel HO, Condy EE, Nguyen T, Blick E, Bress K, Khaksari K, Dashtestani H, Millerhagen J, Shahmohammadi S, Zeytinoglu S, Fox NA, Gandjbakhche A. Cerebral hemodynamic response during a live action-observation and action-execution task: a fNIRS study. PLoS One 2021;16(8):e0253788.
- Nguyen T, Miguel HO, Condy EE, Park S, Gandjbakhche A. Using functional connectivity to examine the correlation between mirror neuron network and autistic traits in a typically developing sample: a fNIRS study. Brain Sci 2021;11(3):397.
- Nguyen T, Khaksari K, Khare SM, Park S, Anderson AA, Bieda J, Jung E, Hsu CD, Romero R, Gandjbakhche AH. Non-invasive transabdominal measurement of placental oxygenation: a step toward continuous monitoring. Biomed Opt Express 2021;12(7):4119–4130.
- Nguyen T, Condy EE, Park S, Friedman BH, Gandjbakhche A. Comparison of functional connectivity in the prefrontal cortex during a simple and an emotional Go/No-Go task in female versus male Groups: an fNIRS study. Brain Sci 2021;11(7):909.
- Park S, Nguyen T, Benoit E, Sackett DL, Garmendia-Cedillos M, Pursley R, Boccara C, Gandjbakhche A. Quantitative evaluation of the dynamic activity of HeLa cells in different viability states using dynamic full-field optical coherence microscopy. Biomed Opt Express 2021;112(10):6431–6441.
Collaborators
- Franck Amyot, PhD, Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD
- Mehran Armand, PhD, The Johns Hopkins University, Baltimore, MD
- Claude Boccara, PhD, École Supérieure de Physique et de Chimie Industrielles, Paris, France
- Nathan Fox, PhD, University of Maryland, College Park, MD
- Andrea Gropman, MD, Children's National Health System, Washington, DC
- Jay Knutson, PhD, Laboratory of Molecular Biophysics, NHLBI, Bethesda, MD
- Tom Pohida, MS, Division of Computational Bioscience, CIT, NIH, Bethesda, MD
- Randall Pursley, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Jessica C. Ramella-Roman, PhD, Florida International University, Miami, FL
- Roberto Romero-Galue, MD, Perinatology Research Branch, NICHD, Detroit, MI
- Dan Sackett, PhD, Division of Basic and Translational Biophysics, NICHD, Bethesda, MD
- Babak Shadgan, MD, MSc, PhD, University of British Columbia, Vancouver, Canada
- Constantine Stratakis, MD, D(med)Sci, Section on Endocrinology and Genetics, NICHD, Bethesda, MD
- Audrey Thurm, PhD, Pediatrics & Developmental Neuropsychiatry Branch, NIMH, Bethesda, MD
- Bruce Tromberg, PhD, Section on Biomedical Optics, NICHD, Bethesda, MD
- Eric Wassermann, MD, Cognitive Neuroscience Section, NINDS, Bethesda, MD
Contact
For more information, email gandjbaa@mail.nih.gov or visit https://irp.nih.gov/pi/amir-gandjbakhche.