Mechanisms of Disease in Preterm Labor and Complications of Prematurity; Prenatal Diagnosis of Congenital Anomalies

- Roberto Romero, MD, DMedSci, Chief, Perinatology Research Branch
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide. The cost of prematurity in the U.S. alone is estimated to be $26 billion per year. An important goal is to understand the mechanisms of disease responsible for spontaneous preterm birth and fetal injury and to develop methods for the prediction and prevention of preterm birth. The Perinatology Research Branch (PRB) proposed that preterm parturition is a syndrome caused by many pathologic processes, i.e., that preterm labor is one syndrome but has many causes. The emphasis of the Branch is to study intra-amniotic infection and inflammation, vascular disorders, maternal anti-fetal rejection (chronic inflammatory lesions of the placenta), cervical disease, and a decline in progesterone action.
However, as has been the case for many clinical and translational research enterprises, an additional focus of ours the last 18 months has been on determining the extent to which the SARS-CoV-2 infection is influencing and potentially exacerbating previously identified causes of preterm birth and other pregnancy complications. We reported that SARS-CoV-2 infection is strongly associated with preeclampsia and preterm birth. Furthermore, SARS-CoV-2 infection in the mother, even in asymptomatic cases, induces unique maternal and fetal inflammatory responses at the maternal-fetal interface (placental tissues) and in the neonate (umbilical cord blood).
In addition to spontaneous preterm birth, the Branch also studies other obstetrical syndromes that account for the high rate of infant mortality in the United States, including clinical chorioamnionitis, which is the most common infection-related diagnosis in delivery units around the world, as well as meconium aspiration syndrome and amniotic fluid embolism.
Congenital anomalies continue to be a leading cause of perinatal mortality in the U.S. Imaging, a powerful tool for scientific discovery, has changed the practice of obstetrics and maternal-fetal medicine. Imaging with ultrasound permits the definition of fetal anatomy, biometry, growth, and the study of physiologic parameters, such as cardiac function, fetal sleep, and breathing.
SARS-CoV-2 infection during pregnancy and the risk of preeclampsia
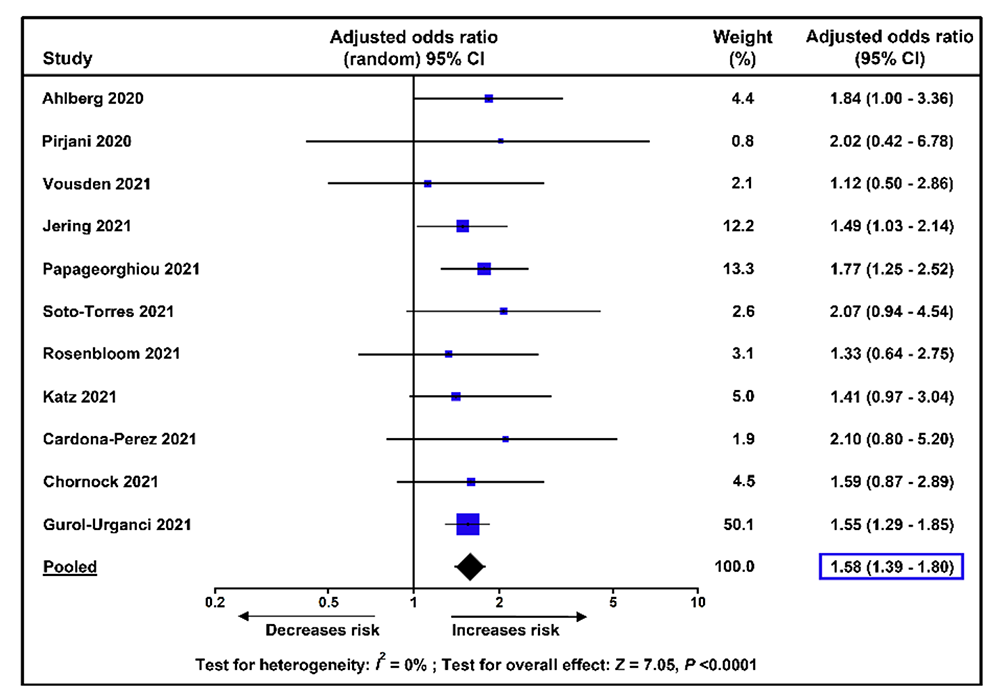
Preeclampsia, a multisystem syndrome that complicates about 5% of pregnancies, is one of the leading causes of maternal mortality worldwide, accounting for approximately 14% of all maternal deaths. During the current pandemic, epidemiological studies have shown that pregnant women with SARS-CoV-2 infection have a significantly higher risk of maternal death, admission to the intensive care unit, preterm birth, and stillbirth than those without SARS-CoV-2 infection. Hence, we performed a systematic review and meta-analysis to assess whether SARS-CoV-2 infection during pregnancy also increases the risk of preeclampsia. A total of 28 studies that included 790,954 pregnant women, among which 15,524 were diagnosed with SARS-CoV-2 infection, met the inclusion criteria. Overall, meta-analyses of unadjusted and adjusted risk estimates showed that pregnant women with SARS-CoV-2 infection had a significantly higher risk of preeclampsia than did pregnant women without SARS-CoV-2 infection. Moreover, there was a significant increase in the odds of preeclampsia with severe features, such as eclampsia and HELLP syndrome, among pregnant women with SARS-CoV-2 infection, as compared with those without the infection. Both asymptomatic and symptomatic SARS-CoV-2 infections significantly increased the odds of preeclampsia, although it was higher among patients with symptomatic illness than among those with asymptomatic illness (Figure 1). We concluded that SARS-CoV-2 infection during pregnancy is associated with higher odds of preeclampsia and hypothesized that this relationship may be causal. The findings of our study have clear implications for patient care, public health policy, and future research.
Figure 1.
Meta-analysis of adjusted odds ratios for the association between SARS-CoV-2 infection during pregnancy and preeclampsia
SARS-COV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose response relationship supporting causality
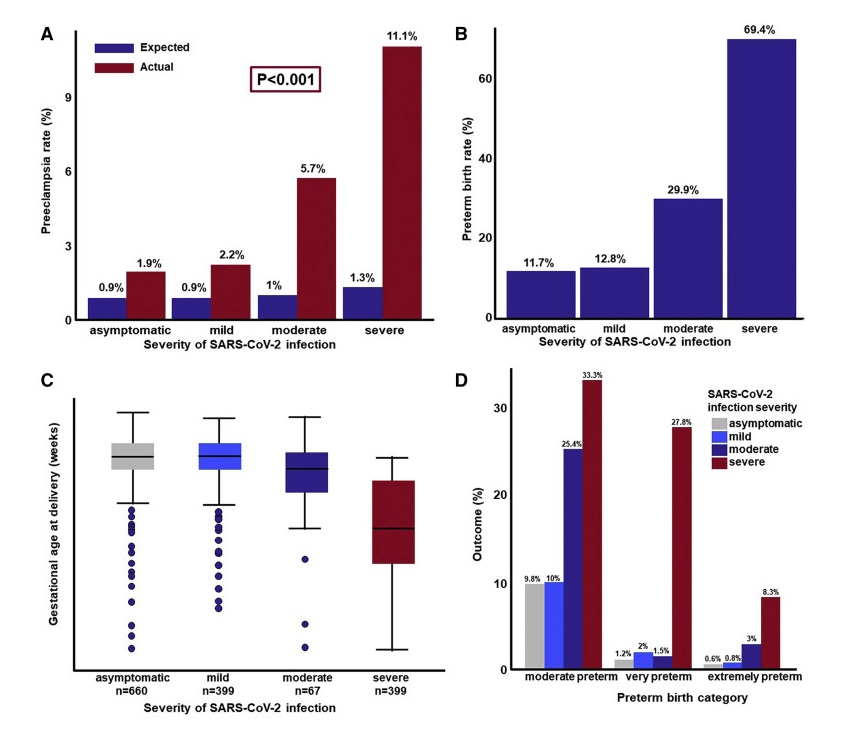
Pregnant women infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have a worse clinical outcome than non-pregnant women infected with SARS-CoV-2. Such adverse outcomes include admission to the intensive care unit, use of invasive mechanical ventilation, and even death. Case series, systematic reviews, and meta-analyses showed an association between SARS-CoV-2 infection and a higher risk for developing preeclampsia; yet, causality needs to be determined. To investigate causality between SARS-CoV-2 infection during pregnancy and preeclampsia, we conducted a retrospective observational study based on data from 14 National Health Service (NHS) maternity hospitals in the United Kingdom, where our collaborators are located. Our study cohort included 1,223 pregnant women with a positive SARS-CoV-2 PCR test. One of the Bradford Hill criteria to assess causality is the existence of a dose-response relationship between exposure and the outcome of interest, in this case, the severity of SARS-CoV-2 infection and the likelihood of developing preeclampsia. The severity of infection with SARS-CoV-2 was defined as asymptomatic, mild, moderate, or severe, and its effect on the rate of preeclampsia and preterm birth was assessed using robust Poisson regression models and chi-square tests for trend. The observed rate of preeclampsia diagnosed at or after SARS-CoV-2 infection was higher than expected (Figure 2A). Patients with severe COVID-19 had a five-fold greater risk of preeclampsia than asymptomatic patients. The relative risk of developing preeclampsia in women with moderate or severe COVID-19 was 3.3-fold higher than in those with asymptomatic/mild infection. The monotonic relationship between the severity of COVID-19 and the risk of developing preeclampsia was significant according to a test for trend. There was also a dose response relationship between the severity of SARS-CoV-2 infection and the risk and severity of preterm birth (Figure 2B-D). In conclusion, the study provides evidence that the more severe the infection with SARS-CoV-2, the greater the risk of preeclampsia and preterm birth. SARS-CoV-2 infection can lead to endothelial dysfunction, intravascular inflammation, proteinuria, activation of thrombin, and hypertension, which are all features of preeclampsia. Therefore, it is likely that SARS-CoV2 infection during pregnancy causes preeclampsia.
Figure 2. Association between SARS-CoV-2 infection severity and pregnancy outcomes
A. Expected and observed rates of preeclampsia in women with SARS-CoV-2 infection.
B. Observed rates of preterm birth in women with SARS-CoV-2 infection who had a live neonate.
C. Gestational age at delivery in women with SARS-CoV-2 infection who had a live neonate.
D. Rate of moderate, very, and extreme preterm birth as a function of the severity of SARS-CoV-2 infection.
Maternal and fetal immune responses in pregnant women infected with SARS-CoV-2
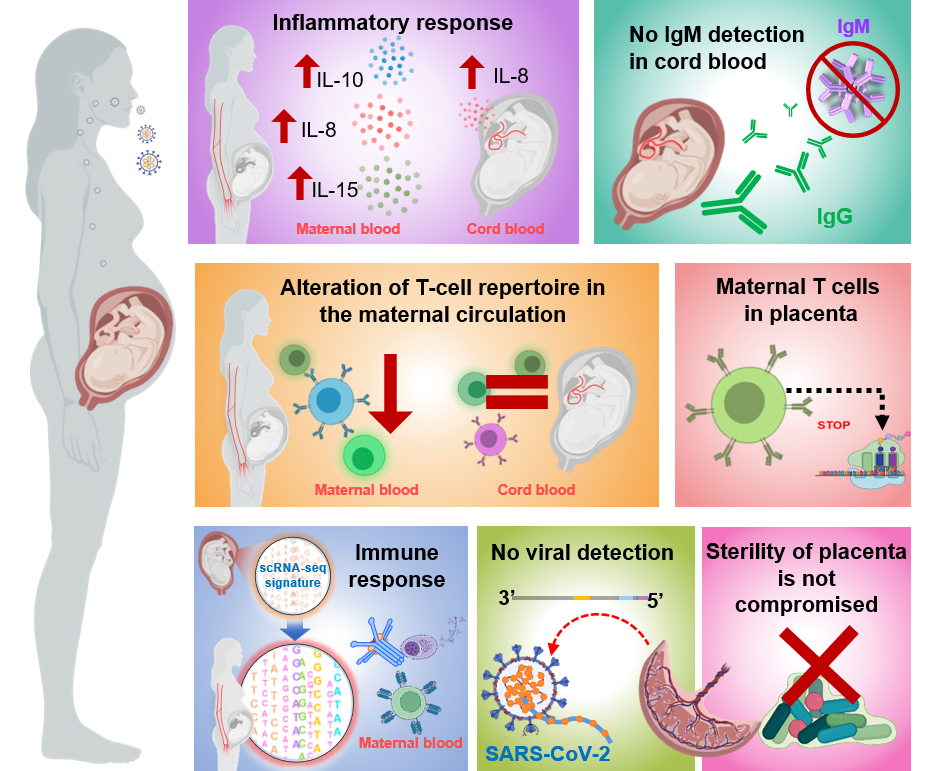
To date, over 118,000 pregnant women in the United States have been infected with SARS-CoV-2, the virus responsible for the coronavirus disease 2019 (COVID-19). Pregnant women with SARS-CoV-2 infection are at a high risk for severe/critical disease and mortality as well as preterm birth. Therefore, we investigated the host immune responses in pregnant women infected with SARS-CoV-2, even if they were asymptomatic. The studies comprise a multidisciplinary approach including the detection of SARS-CoV-2 IgM/IgG, multiplex cytokine assays, immunophenotyping, single-cell and bulk RNA sequencing, and viral RNA and protein detection (Figure 3), together with the assessment of the microbiome diversity and histopathology of the placenta, to characterize the maternal-fetal immune responses triggered by SARS-CoV-2 during pregnancy. We gathered evidence showing that SARS-CoV-2 infection during pregnancy primarily induced unique inflammatory responses in the circulation and at the maternal-fetal interface, with the latter being governed by maternal T cells and fetal stromal cells. SARS-CoV-2 infection during pregnancy is also associated with a mild cytokine response in the fetal circulation (i.e., umbilical cord blood) without compromising the T-cell repertoire or initiating IgM responses. Moreover, bulk RNA sequencing of maternal and cord blood revealed that SARS-CoV-2 infection differentially impacts the transcriptome of the mother and neonate, and correlation analyses between bulk RNA-Seq blood data and single-cell placental data, indicated that maternal and neonatal transcriptomic changes are partly shared with those in the placental tissues. Importantly, SARS-CoV-2 has not been detected in the placental tissues, nor was the sterility of the placenta compromised by maternal viral infection. The study provides insight into the maternal-fetal immune responses triggered by SARS-CoV-2 and further emphasizes the rarity of placental infection.
Figure 3. Maternal and fetal immune responses in pregnant women infected with SARS-CoV-2
Overall study design showing the detection of SARS-CoV-2 lgM/lgG, multiplex cytokine assays, immuno-phenotyping, single-cell transcriptomics, and viral RNA and protein detection that were applied to investigate host immune responses in pregnant women infected with SARS-CoV-2.
Crowdsourcing assessment of maternal blood multi-omics for predicting gestational age and preterm birth
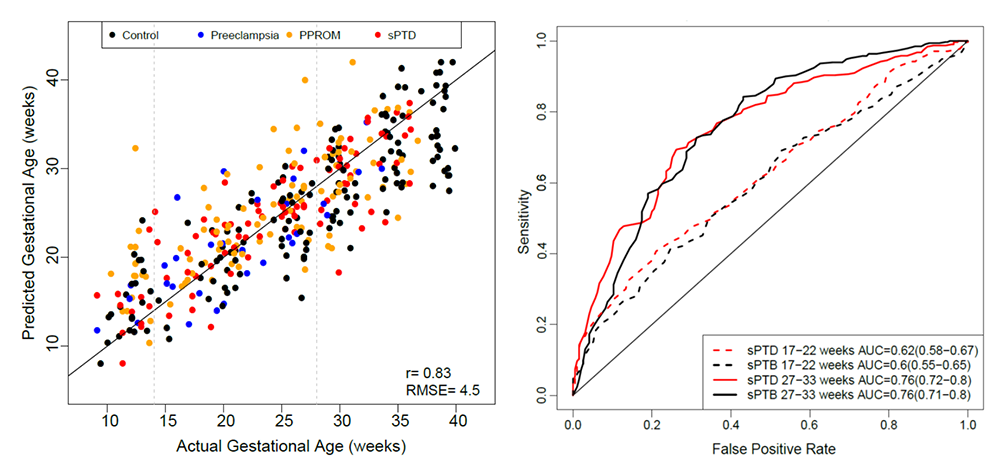
Identification of pregnancies at risk of preterm birth, the leading cause of newborn deaths, remains challenging given the syndromic nature of the disease. We reported a longitudinal multi-omics study coupled with a crowdsourcing initiative (DREAM challenge) to develop predictive models of gestational age and spontaneous preterm birth. The computational challenge was based on previously published and new data the Branch generated, while incentives for participating in the challenge were funded by Wayne State University and the March of Dimes. The findings indicate that whole-blood gene expression predicts ultrasound-based gestational ages at blood draw in normal and complicated pregnancies. Based on samples collected before 33 weeks in asymptomatic women, our analysis suggests that gene-expression changes preceding preterm prelabor rupture of the membranes are consistent across time points and cohorts, and involve leukocyte-mediated immunity. Models built from plasma proteomic data predict spontaneous preterm delivery with intact membranes with higher accuracy and earlier in pregnancy than transcriptomic models (Figure 4). The work therefore identified RNAs and proteins in the maternal circulation predictive of spontaneous preterm birth in asymptomatic patients that can be further evaluated in larger targeted studies. Early prediction of women at risk could enable preventive strategies. Moreover, a byproduct of the study is a suite of methods and software tools for longitudinal omics data that can be applied to the study of other adverse pregnancy outcomes to identify biomarkers. These methods are available at https://www.synapse.org/pretermbirth.
Figure 4. Prediction of gestational age and preterm birth using maternal blood omics data
The figure shows blood RNA–based predicted gestational ages in normal and complicated pregnancies versus the actual values (left), and sensitivity of plasma protein models as a function of false positive rate for prediction of preterm birth based on samples collected at 17–22 and 27–33 weeks in asymptomatic women (right). sPTB: spontaneous preterm birth; sPTD; spontaneous preterm birth with intact membranes; PPROM: preterm, prelabor rupture of membranes.
The amniotic fluid cell-free transcriptome in spontaneous preterm labor
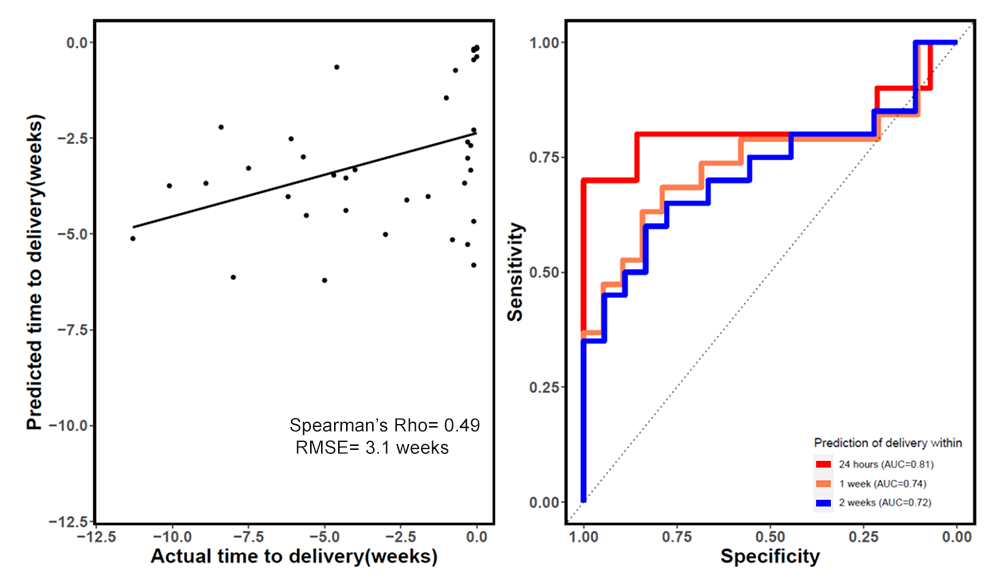
Amniotic fluid cell-free RNA was shown to reflect physiological and pathological processes in pregnancy, but its value in the prediction of spontaneous preterm delivery is unknown. We therefore profiled cell-free RNA in amniotic fluid samples collected from women who underwent transabdominal amniocentesis after an episode of spontaneous preterm labor and subsequently delivered within 24h or later in gestation. Expression of known placental single-cell RNA-Seq signatures was quantified in amniotic fluid cell-free RNA and compared between the groups. We applied random forest models to predict time-to-delivery after amniocentesis. We found 2,385 genes differentially expressed in samples of women who delivered within 24 h of amniocentesis compared with gestational age-matched samples from women who delivered 24 h after amniocentesis. Genes with cell-free RNA changes were associated with immune and inflammatory processes related to the onset of labor, and the expression of placental single-cell RNA-Seq signatures of immune cells was increased with imminent delivery. Transcriptomic prediction models captured these effects and predicted delivery within 24 h of amniocentesis (Figure 5). The results may inform the development of biomarkers for spontaneous preterm birth.
Figure 5. Prediction of time to delivery after an episode of preterm labor
The figure shows amniotic fluid cell free RNA–predicted time from amniocentesis to delivery versus actual time to delivery (left) and sensitivity versus specificity of RNA–based prediction of imminent delivery (within 24h, 1 week, or 2 weeks) (right).
Clinical chorioamnionitis
Clinical chorioamnionitis at term is considered the most common infection-related diagnosis in labor and delivery units worldwide. The syndrome affects 5–12% of all term pregnancies and is a leading cause of maternal morbidity and mortality as well as neonatal death and sepsis. We found that: (1) intra-amniotic infection (defined as the combination of microorganisms detected in amniotic fluid and an elevated IL-6 concentration) was present in 63% of cases; (2) the most common microorganisms found in the amniotic fluid samples were Ureaplasma species, followed by Gardnerella vaginalis; (3) sterile intra-amniotic inflammation (elevated IL-6 in amniotic fluid but without detectable microorganisms) was present in 5% of cases; (4) 26% of patients with the diagnosis of clinical chorioamnionitis had no evidence of intra-amniotic infection or intra-amniotic inflammation; (5) intra-amniotic infection was more common when the membranes were ruptured than when they were intact (78% vs. 38%); (6) the traditional criteria for the diagnosis of clinical chorioamnionitis had poor diagnostic performance in identifying proven intra-amniotic infection (overall accuracy, 40–58%); (7) neonatal bacteremia was diagnosed in 4.9% of cases; and (8) a fetal inflammatory response defined as the presence of severe acute funisitis was observed in 33% of cases. These observations have implications for the diagnosis, treatment, and optimal management of the neonate born to mothers with the most common infection in obstetrics.
Resolution of acute cervical insufficiency after antibiotics
Cervical insufficiency generally refers to a condition in which there is mid-trimester cervical dilatation or protruding chorioamniotic membranes in the absence of uterine contractions. The condition is a risk factor for mid-trimester spontaneous abortion or early preterm birth, and is associated with adverse neonatal outcomes. Both intra-amniotic infection and inflammation, ascertained by amniocentesis, have been identified in patients with cervical insufficiency, and are poor prognostic factors. A subset of patients with intra-amniotic inflammation will have no demonstrable microorganisms detected via cultivation or molecular methods, and therefore represent cases of sterile intra-amniotic inflammation. Amniotic fluid sludge (free-floating hyperechogenic material within the amniotic fluid in close proximity to the uterine cervix) identified on sonography is a biomarker for intra-amniotic infection and inflammation. Recent evidence suggests that intra-amniotic infection, as well as sterile intra-amniotic inflammation, can be treated successfully using antimicrobial agents. We reported a unique case in which administration of antibiotics in the presence of mid-trimester cervical insufficiency, sterile intra-amniotic inflammation, and amniotic fluid sludge was associated with resolution of the cervical findings (as demonstrated on both sonographic and speculum examination). The patient successfully underwent elective cesarean delivery at 36 2/7 weeks of gestation. The case illustrates that antibiotic therapy may be effective despite the presence of several high-risk pregnancy conditions, and that successful outcome is possible.
Cardiac measurements of size and shape in fetuses with absent or reversed end-diastolic velocity of the umbilical artery and perinatal survival and severe growth restriction before 34 weeks' gestation
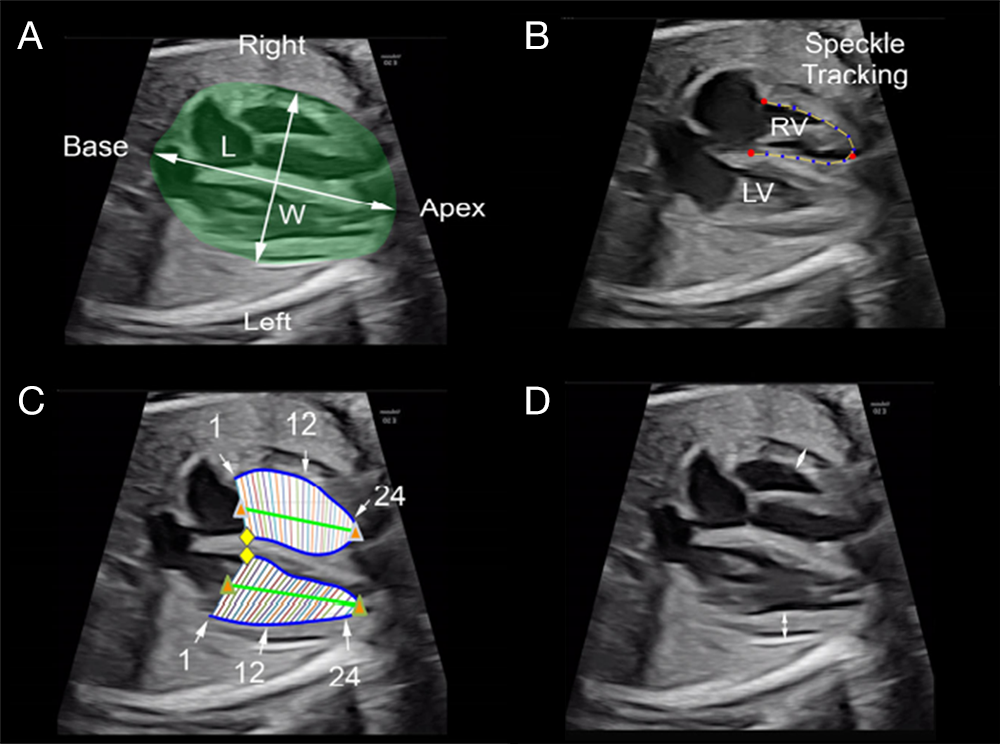
The purpose of this study was to evaluate the end-diastolic size and shape of the 4-chamber view as well as the right ventricle (RV) and left ventricle (LV) in growth-restricted fetuses before 34 weeks' gestation with absent or reversed end-diastolic velocity of the umbilical artery and to compare the results between those with perinatal deaths with those who survived the neonatal period. Of the 49 fetuses, there were 13 perinatal deaths (27%) and 36 (63%) neonatal survivors. Measurements that were unique for neonatal survivors were an increased RV apical transverse width and decreased measurements of the following: LV and RV widths, LV and RV areas, as well as RV sphericity indices (Figure 6). We concluded that fetuses with a smaller RV and LV size and area and those with a globular-shaped RV were at significantly lower risk for perinatal death.
Figure 6. Measurements of the 4CV, RV, and LV
A. End-diastolic length (L) and width (W) of the 4CV measured at the point of the greatest length and width.
B. Speckle-tracking contour from the RV at end diastole.
C. Twenty-four–segment transverse widths and ventricular length computed from the speckle-tracking analysis of each ventricular chamber. Numbers identify segments 1, 12, and 24.
D. Measurements of ventricular wall thickness (double arrows) from the midsection of the RV and LV. 4CV: four chamber view; RV: right ventricle; LV: left ventricle.
Additional Funding
- NICHD/NIH/DHHS
Publications
- Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol 2022;226(1):68–89.e3.
- Lai J, Romero R, Tarca AL, Iliodromiti S, Rehal A, Banerjee A, Yu C, Peeva G, Palaniappan V, Tan L, Mehta M, Nicolaides KH. SARS-CoV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose-response relationship supporting causality. Am J Obstet Gynecol 2021;225(6):689–693.
- Garcia-Flores V, Romero R, Xu Y, Theis KR, Arenas-Hernandez M, Miller D, Peyvandipour A, Bhatti G, Galaz J, Gershater M, Leneson D, Pusod E, Tao L, Kracht D, Florova V, Leng Y, Motomura K, Para R, Faucett M, Hsu CD, Zhang G, Tarca AL, Pique-Regi R, Gomez-Lopez N. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun 2022;13(1):320.
- Tarca AL, Pataki BÁ, Romero R, Sirota M, Guan Y, Kutum R, Gomez-Lopez N, Done B, Bhatti G, Yu T, Andreoletti G, Chaiworapongsa T; DREAM Preterm Birth Prediction Challenge Consortium, Hassan SS, Hsu CD, Aghaeepour N, Stolovitzky G, Csabai I, Costello JC. Crowdsourcing assessment of maternal blood multi-omics for predicting gestational age and preterm birth. Cell Rep Med 2021;2:100323.
- Bhatti G, Romero R, Gomez-Lopez N, Pique-Regi R, Pacora P, Jung E, Yeo L, Hsu CD, Kavdia M, Tarca AL. The amniotic fluid cell-free transcriptome in spontaneous preterm labor. Sci Rep 2021;13481.
- Romero R, Pacora P, Kusanovic JP, Jung E, Panaitescu B, Maymon E, Erez O, Berman S, Bryant DR, Gomez-Lopez N, Theis KR, Bhatti G, Kim CJ, Yoon BH, Hassan SS, Hsu CD, Yeo L, Diaz-Primera R, Marin-Concha J, Lannaman K, Alhousseini A, Gomez-Roberts H, Varrey A, Garcia-Sanchez A, Gervasi MT. Clinical chorioamnionitis at term X: microbiology, clinical signs, placental pathology, and neonatal bacteremia—implications for clinical care. J Perinat Med 2021;49:275–298.
Collaborators
- Tinnakorn Chaiworapongsa, MD, Wayne State University School of Medicine, Detroit, MI
- Agustin Conde-Agudelo, MD, Wayne State University School of Medicine, Detroit, MI
- Mark Haacke, PhD, Wayne State University School of Medicine, Detroit, MI
- Leonid Margolis, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Adi L. Tarca, PhD, Wayne State University, Detroit Medical Center, Detroit, MI
- Lami Yeo, MD, Wayne State University School of Medicine, Detroit, MI
- Bo Hyun Yoon, MD, PhD, Seoul National University, Seoul, Korea
Contact
For more information, email romeror@mail.nih.gov or visit https://irp.nih.gov/pi/roberto-romero.