Protein Sorting in the Endosomal-Lysosomal System

- Juan S. Bonifacino, PhD, Head, Section on Intracellular Protein Trafficking
- Rafael Mattera, PhD, Staff Scientist
- Yu Chen, PhD, Research Fellow
- Loreto Cuitiño, PhD, Visiting Fellow
- Ginny Farias, PhD, Visiting Fellow
- David Gershlick, PhD, Visiting Fellow
- Carlos M. Guardia, PhD, Visiting Fellow
- Xiaoli Guo, PhD, Visiting Fellow
- Rui Jia, PhD, Visiting Fellow
- Tal Keren-Kaplan, PhD, Visiting Fellow
- Sang-Yoon Park, PhD, Visiting Fellow
- Jing Pu, PhD, Visiting Fellow
- Dylan Britt, BSc, Postbaccalaureate Student
- Xiaolin Zhu, RN, Technician
We investigate the molecular mechanisms by which transmembrane proteins (referred to as cargo) are sorted to different compartments of the endomembrane system in eukaryotic cells. The system consists of an array of membrane-enclosed organelles including the endoplasmic reticulum (ER), the Golgi apparatus, the trans-Golgi network (TGN), endosomes, lysosomes, lysosome-related organelles (LROs) (e.g., melanosomes), and various domains of the plasma membrane in polarized cells (e.g., epithelial cells and neurons). Transport of cargo between these compartments is mediated by carrier vesicles or tubules that bud from a donor compartment, translocate through the cytoplasm, and eventually fuse with an acceptor compartment. Work in our laboratory focuses on the molecular machineries that mediate these processes, including (1) sorting signals and adaptor proteins that select cargo proteins for packaging into the transport carriers, (2) microtubule motors that drive movement of the transport carriers and other organelles through the cytoplasm, and (3) tethering factors that promote fusion of the transport carriers to acceptor compartments. We study the machineries in the context of different intracellular transport pathways, including endocytosis, recycling to the plasma membrane, retrograde transport from endosomes to the TGN, biogenesis of lysosomes and LROs, and polarized sorting in epithelial cells and neurons. We apply knowledge gained from our research to the elucidation of disease mechanisms, including congenital disorders of protein traffic such as the pigmentation and bleeding disorder Hermansky-Pudlak syndrome (HPS) and the neuro-cutaneous disorder MEDNIK syndrome. In addition, we study how the molecular mechanisms of protein transport are exploited by intracellular pathogens such as HIV-1.
An AP-1/clathrin pathway for the sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons
A major focus of our research is processes mediated by recognition of sorting signals in the cytosolic tails of transmembrane proteins by adaptor proteins that are components of protein coats (e.g., clathrin coats). Two types of sorting signal—tyrosine-based and dileucine-based—participate in various sorting events, including endocytosis, transport to lysosomes and melanosomes, and sorting to the basolateral surface of polarized epithelial cells. In previous work, we found that tyrosine-based signals bind to a conserved site on the mu1, mu2, and mu3 subunits of three hetero-tetrameric adaptor protein (AP) complexes, AP-1, AP-2, and AP-3, respectively. Dileucine-based signals, on the other hand, bind to a different site, a site that spans the surface of two subunits—in the case of the AP-1 complex the gamma-sigma1 subunits, of the AP-2 complex the alpha-sigma2 subunits, and of the AP-3 complex the delta-sigma3 subunits. In recent years, we extended our studies to the role of signal-adaptor interactions in the process of polarized sorting in neurons. Neurons are highly polarized cells with distinct somatodendritic and axonal domains. The plasma membrane of each of these domains possesses a distinct set of transmembrane proteins, including receptors, channels, transporters, and adhesion molecules. We found that several transmembrane proteins, including the transferrin receptor (TfR), the Coxsackie virus and adenovirus receptor (CAR), and the Nipah virus fusion glycoprotein (NiV-F), are sorted to the somatodendritic domain by interaction of tyrosine-based signals with the mu1A subunit of AP-1. More recently, we discovered that a different set of proteins, including the copper transporter ATP7B and the SNARE VAMP4, also undergo sorting to the somatodendritic domain, but in this case through recognition of dileucine-based signals by the gamma1-sigma1 subunits of AP-1. Together with previous work on epithelial cells, these findings establish the AP-1 complex as a global regulator of polarized sorting in different cell types. Defects in polarized sorting likely underlie the pathogenesis of several neuro-cutaneous disorders caused by mutation in sigma1 subunit isoforms, such as the MEDNIK syndrome (sigma1A), Fried/Pettigrew syndrome (sigma1B), and pustular psoriasis (sigma1C).
Sorting of dendritic and axonal vesicles at the pre-axonal exclusion zone (PAEZ)
Polarized sorting of newly-synthesized proteins to the somatodendritic and axonal domains of neurons occurs by selective incorporation into distinct populations of vesicular transport carriers. An unresolved issue is how the vesicles themselves become sorted to their corresponding neuronal domains. Previous studies led to the conclusion that the axon initial segment (AIS) is an actin-based filter that selectively prevents passage of somatodendritic vesicles into the axon. We found, however, that most somatodendritic vesicles fail to enter the axon at a more proximal region in the axon hillock named the “pre-axonal exclusion zone” (PAEZ). Forced coupling of a somatodendritic cargo protein to an axonally directed kinesin is sufficient to drive transport of whole somatodendritic vesicles through the PAEZ toward the distal axon. Based on these findings, we proposed that polarized sorting of transport vesicles occurs at the PAEZ and depends on the ability of the vesicles to acquire an appropriately directed microtubule motor.
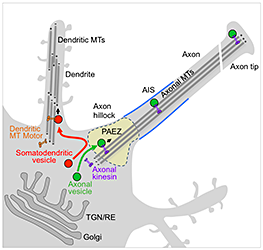
Click image to enlarge.
Figure 1. Sorting of somatodendritic and axonal vesicles at the pre-axonal exclusion zone (PAEZ)
BORC: a novel multisubunit complex that regulates lysosome positioning and motility
In another line of research, we recently obtained unexpected insights into the mechanisms of lysosome positioning and motility. The research was an outgrowth of our previous work on the biogenesis of LROs such as melanosomes. Years ago, we discovered that mutations in AP-3 cause eye color defects in Drosophila and the pigmentation and bleeding disorder Hermansky-Pudlak syndrome (HPS) type 2 (HPS-2) in humans. Other types of HPS are caused by mutations in subunits of the hetero-octameric BLOC-1 and the heterodimeric BLOC-3 complexes. AP-3 mediates sorting of transmembrane proteins to melanosomes, but the functions of BLOC-1 and BLOC-3 are less well understood. In experiments aimed at identifying proteins that interact with BLOC-1, we made the surprising discovery of a related hetero-octameric complex named BORC (for BLOC-one-related complex). Biochemical analyses showed that BORC comprises three subunits that are shared with BLOC-1 (named BLOS1, BLOS2, and Snapin) and five unique subunits (named KXD1, MEF2B, Myrlysin, Lyspersin, and Diaskedin). Further studies revealed that BORC is associated with late endosomes and lysosomes, where it functions to recruit the small GTPase Arl8, which initiates a chain of interactions that drives kinesin-dependent movement of lysosomes toward the peripheral cytoplasm. Mutations in BORC cause collapse of the lysosomal population into the pericentrosomal area. In addition, BORC–mutant cells exhibit defective autophagic flux, probably a result of the inability of lysosomes to reach autophagosomes in the cell periphery. The cells also display reduced spreading and migration, likely caused by impaired lysosome-dependent delivery of adhesion and signaling molecules to the plasma membrane. Given the critical importance of cell adhesion and motility in tumor growth, invasion, and metastasis, the BORC pathway of lysosome dispersal could be an attractive target for pharmacologic inhibition in cancer therapeutics.
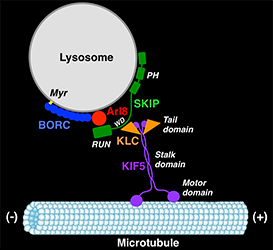
Click image to enlarge.
Figure 2. BORC regulates kinesin-dependent movement of lysosomes to the cell periphery.
GARP and EARP: multisubunit tethering complexes involved in endosomal retrieval pathways
Recycling of endocytic receptors to the cell surface involves passage through a series of membrane-bound compartments by mechanisms that are poorly understood. In particular, prior to our work, it was unknown whether endocytic recycling requires the function of multisubunit tethering complexes, as is the case for other intracellular trafficking pathways. In the course of studies on the Golgi-associated retrograde protein (GARP) complex, we discovered a related complex named endosome-associated recycling protein (EARP). The two complexes share the Ang2 (also known as Vps51), Vps52, and Vps53 subunits, but whereas GARP comprises a fourth subunit named Vps54, EARP contains a previously uncharacterized protein named Syndetin, a divergence that determines differential localization of GARP to the TGN and EARP to recycling endosomes. Importantly, we found that EARP is involved in recycling of internalized proteins to the plasma membrane. The findings should contribute to the understanding of the pathogenesis of progressive cerebello-cerebral atrophy type 2, a neurodegenerative disorder caused by mutations in Vps53, which in light of our results could result from impairment of both GARP–mediated retrograde transport to the TGN and EARP–mediated recycling to the plasma membrane.
Mechanisms of CD4 downregulation by HIV-1 Nef
In previous work, we found that the HIV-1 accessory protein Nef connects surface CD4 to both the endocytic and lysosomal targeting machineries, leading to efficient and sustained removal of CD4 from host cells early during infection. The role of Nef in CD4 internalization involves an interaction with the AP-2 clathrin adaptor. Subsequent to induction of CD4 internalization by an AP-2/clathrin pathway, Nef promotes delivery of internalized CD4 to the multivesicular body pathway (MVB) for eventual degradation in lysosomes. In collaboration with Luis daSilva, we recently found that this targeting depends on a direct interaction of Nef with Alix/AIP1, a protein associated with the Endosomal Sorting Complexes Required for Transport (ESCRT) machinery, which assists with cargo recruitment and intraluminal vesicle formation in MVBs. We showed that Nef interacts with both the Bro1 and V domains of Alix. Depletion of Alix or overexpression of the Alix V domain impaired lysosomal degradation of CD4 induced by Nef. In contrast, V-domain overexpression did not prevent cell surface removal of CD4 by Nef or protein targeting to the canonical, ubiquitination-dependent MVB pathway. We also showed that the Nef-Alix interaction occurs in late endosomes that are enriched in internalized CD4. Together, the results indicated that Alix functions as an adaptor for the ESCRT–dependent, ubiquitin-independent targeting of CD4 to the MVB pathway induced by Nef.
Additional Funding
- Intramural AIDS Targeted Antiviral Program (IATAP)
Publications
- Mattera R, Farías GG, Mardones GA, Bonifacino JS. Co-assembly of viral envelope glycoproteins regulates their polarized sorting in neurons. PLoS Pathogens 2014; 10:e1004107.
- Jain S, Farías GG, Bonifacino JS. Polarized sorting of the copper transporter ATP7B in neurons mediated by recognition of a dileucine signal by AP-1. Mol Biol Cell 2015; 26:218-228.
- Pu J, Schindler C, Jia R, Jarnik M, Backlund P, Bonifacino JS. BORC, a multisubunit complex that regulates lysosome positioning. Dev Cell 2015; 33:176-188.
- Schindler C, Chen Y, Pu J, Guo X, Bonifacino JS. EARP is a multisubunit tethering complex involved in endocytic recycling. Nat Cell Biol 2015; 17:639-650.
- Farias GG, Guardia CM, Britt DJ, Guo X, Bonifacino JS. Sorting of dendritic and axonal vesicles at the pre-axonal exclusion zone. Cell Rep 2015; E-pub ahead of print.
Collaborators
- Luis L. P. DaSilva, PhD, University of São Paulo, São Paulo, Brazil
- Eric O. Freed, PhD, HIV Drug Resistance Program, Center for Cancer Research, NCI, Frederick, MD
- Aitor Hierro, PhD, CIC-bioGUNE, Bilbao, Spain
- James Hurley, PhD, Laboratory of Molecular Biology, NIDDK, Bethesda, MD (now at now at UC Berkeley, CA)
- Abdul Waheed, PhD, Retroviral Replication Laboratory, Center for Cancer Research, NCI, Frederick, MD
Contact
For more information, email bonifacinoj@helix.nih.gov or visit http://cbmp.nichd.nih.gov/sipt.


