Diagnosis, Localization, Pathophysiology, and Molecular Biology of Pheochromocytoma and Paraganglioma

- Karel Pacak, MD, PhD, DSc, Head, Section on Medical Neuroendocrinology
- Thanh-Truc Huynh, MS, Biologist
- Karen T. Adams, MSc, CRNP, Research Nurse
- Ingo Janssen, MD, Postdoctoral Visiting Fellow
- Petra Bullova, MS, Predoctoral Visiting Fellow
- Ivana Jochmanova, MS, Predoctoral Visiting Fellow
- Joan Nambuba, BA, Postbaccalaureate Fellow
- Roland Darr, MD, Volunteer
We conduct patient-oriented research into the etiology, pathophysiology, genetics, diagnosis, localization, and treatment of pheochromocytoma and paraganglioma. Projects include both translational research—applying basic science knowledge to clinical diagnosis, pathophysiology, and treatment—and 'reverse translation research,' by which clinical findings lead to new concepts for pursuit by basic researchers in the laboratory. Our goals are to (1) establish new and improved methods and strategies for novel diagnostic and localization approaches to pheochromocytoma/paraganglioma; (2) explain the molecular and cellular basis for varying clinical presentations of pheochromocytomas/paragangliomas and establish the pathways of tumorigenesis; (3) search for new molecular and genetic markers for diagnosis and treatment of metastatic pheochromocytoma/paraganglioma; (4) introduce new therapeutic options for malignant/metastatic pheochromocytoma/paraganglioma; and (5) facilitate new and improved collaborations and interdisciplinary studies. To achieve these goals, we enter into multidisciplinary collaborations with investigators from several NIH Institutes and outside medical centers. We link a patient-oriented component with two bench-level components. The patient-oriented component (medical neuroendocrinology) is the driving force for our hypotheses and discoveries. The two bench-level components (tumor pathogenesis/genetics and chemistry; biomarkers) emphasize, first, technologies of basic research tailored for pathway and target discovery and, second, the further development of discoveries into clinical applications.
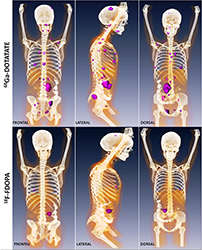
Click image to enlarge.
Metastatic paraganglioma detected by 68Ga-DOTATATE PET/CT and 18F-FDOPA PET/CT
Detection of metastatic paraganglioma with the novel imaging modality 68Ga-DOTATATE PET/CT compared with 18F-FDOPA PET/CT (frontal, lateral, and dorsal views)
Authors: Papadakis GZ, Bagci U, Millo CM, Janssen I, Patronas NJ, Stratakis CA, Pacak K
Clinical, biochemical, and metabolomic aspects of pheochromocytoma and paraganglioma
Testing for mutations in SDHB, the gene encoding a subunit of the mitochondrial enzyme succinate dehdrogenase (SDHx), is recommended in all patients with metastatic pheochromocytoma/paraganglioma (PHEO/PGL), but may not be required when metastatic disease is accompanied by adrenaline production. A retrospective cohort study aimed to establish the prevalence of SDHB mutations among patients with metastatic PHEO/PGL characterized by production of adrenaline compared with those without production of adrenaline, and to establish genotype-phenotype features of metastatic PHEOs/PGLs according to underlying gene mutations. 205 patients (114 males) age (range 9 to 86 years) at the diagnosis of metastatic PHEO/PGL, with and without adrenaline production, were tested for the presence of SDHB mutations or deletions. Twenty-three of the 205 patients (11%) had disease characterized by production of adrenaline, as defined by increased plasma concentrations of metanephrine greater than 5% of the combined increase of both normetanephrine and metanephrine. None of the 23 patients had SDHB mutations. Of the other 182 patients with no tumoral adrenaline production, 51% had SDHB mutations. Metastases in the bone were 36% to 41% more prevalent among patients with SDHB mutations or extra-adrenal primary tumors than those without mutations or with adrenal primary tumors. Liver metastases were 81% more prevalent among patients with adrenal than extra-adrenal primary tumors. SDHB mutation testing has no utility among patients with adrenaline-producing metastatic PHEOs/PGLs, but is indicated in other patients with metastatic disease. Our study also reveals novel associations of metastatic spread with primary tumor location and the presence of SDHB mutations.
Mutations of SDHx genes increase susceptibility to development of PHEOs/PGLs, with particularly high rates of malignancy associated with SDHB mutations. We assessed whether altered succinate dehydrogenase product-precursor relationships, manifested by differences in tumor ratios of succinate to fumarate or other metabolites, might aid in identifying and stratifying patients with SDHx mutations. PHEO/PGL tumor specimens from 233 patients, including 45 with SDHx mutations, were provided from eight tertiary referral centers for mass-spectrometric analyses of Krebs cycle metabolites. Diagnostic performance of the succinate:fumarate ratio was used for the identification of pathogenic SDHx mutations. SDH–deficient PHEOs/PGLs were characterized by 25-fold higher succinate and 80% lower fumarate, cis-aconitate, and isocitrate tissue levels than PHEOs/PGLs without SDHx mutations. Receiver-operating characteristic curves for the use of succinate to fumarate or to cis-aconitate and isocitrate ratios to identify SDHx mutations indicated areas under the curves of 0.94 to 0.96; an optimal cut-off of 97.7 for the succinate:fumarate ratio provided a diagnostic sensitivity of 93% at a specificity of 97% to identify SDHx–mutated PHEOs/PGLs. Succinate:fumarate ratios were higher in both SDHB–mutated and metastatic tumors than in those due to SDHD/C mutations or without metastases. Mass spectrometric-based measurements of ratios of succinate:fumarate and other metabolites in PHEOs/PGLs offer a useful method to identify patients for testing of SDHx mutations, with additional utility to quantitatively assess functionality of mutations and metabolic factors responsible for malignant risk.
The present study investigated the impact of genetic alterations on metabolic networks in PGLs. Homogenates of 32 sporadic PGLs and 48 PGLs from patients with mutations in genes encoding SDHB, SDHD, SDHAF-2, von Hippel–Lindau tumor suppressor (VHL), RET, and NF-1 were subjected to proton nuclear magnetic resonance (1H-NMR) spectroscopy at 500 MHz for untargeted and HPLC tandem mass spectrometry for targeted metabolite profiling. 1H-NMR spectroscopy identified 28 metabolites in PGLs, of which 12 showed genotype-specific differences. Part of these results were published earlier and reported significantly low complex II activity and significantly low ATP/ADP/AMP content in SDH–related PGLs compared with sporadics and PGLs of other genotypes. Extending these results, we observed significantly lower levels of N-acetylaspartic acid (NAA) in SDH tumors and creatine in VHL tumors than in sporadics and other genotypes. A positive correlation was observed between NAA and ATP/ADP/AMP content and NAA and complex II activity of PGLs. Targeted purine analysis in PHEOs/PGLs showed significantly lower adenine in cluster 1 than in cluster 2 tumors (see next section), while significantly lower levels of guanosine and hypoxanthine were observed in RET tumors than in SDH tumors. Principal component analysis (PCA) of metabolites could distinguish PGLs of different genotypes. The present study gives a comprehensive picture of alterations in energy metabolism in SDH– and VHL–related PGLs and establishes the inter-relationship between energy metabolism and amino acid and purine metabolism in PGLs.
One hundred and six patients with SDHB mutation–related PHEO/PGL were included in this retrospective study. We analyzed the recorded largest diameters, locations, and patient ages at initial diagnosis of SDHB–related primary tumors in the context of time-to-metastasis and patient survival. First, the development of metastatic disease in patients with primary tumors measuring 4.5 cm or greater was significantly earlier than in patients with smaller tumors. Second, patients with primary tumors larger than 5.5 cm also had worse overall survival rates than patients with smaller tumors. Third, age at initial diagnosis was found to be an independent predictor of patient survival. Fourth, we did not observe a significant difference in survival based on the specific SDHB mutations or patient sex. Receiver-operating-characteristic curves established 4.5 cm as the best value to dichotomize the primary SDHB–related PHEO/PGL in order to evaluate the development of metastatic disease and 5.5 cm as the best value for survival prediction. Subsequently, we found the size of the primary tumor to be an age-independent predictor of patient survival and metastases development in PGL. In both PHEO and PGL, age at diagnosis was found to be a size-independent predictor of patient survival. We found no significant difference in metastases development or patient survival between males and females or among specific SDHB mutations. The data further extend and support previous recommendations that carriers with SDHB mutations must undergo early and regular evaluations to detect PHEO/PGL in order to achieve the best clinical outcome.
Hereditary pheochromocytoma and paraganglioma
Many solid tumors, including PHEO and PGL, are characterized by a (pseudo)hypoxic signature. (Pseudo)hypoxia has been shown to promote both tumor progression and resistance to therapy. The major mediators of the transcriptional hypoxic response are hypoxia-inducible factors (HIFs) (Reference 1). High levels of HIFs lead to transcription of hypoxia-responsive genes, which are involved in tumorigenesis. PHEOs and PGLs are catecholamine-producing tumors arising from sympathetic- or parasympathetic-derived chromaffin tissue. In recent years, substantial progress has been made in understanding the metabolic disturbances present in PHEO and PGL, especially as a result of the identification of some disease-susceptibility genes. To date, nineteen PHEO and PGL susceptibility genes have been identified. Based on the main transcription signatures of the mutated genes, PHEOs and PGLs were divided into two clusters: pseudohypoxic cluster 1 and pseudohypoxic cluster 2 that is rich in kinase receptor signaling and protein translation pathways. Although the two clusters appear to show distinct signaling pathways, recent data suggest that both clusters are interconnected by HIF signaling as the important driver in their tumorigenesis, and mutations in most PHEO– and PGL–susceptibility genes seem to affect HIFα regulation and its downstream signaling pathways. HIF signaling appears to play an important role in the development and growth of PHEOs and PGLs, which could suggest new therapeutic approaches for the treatment of these tumors
Previously, no HIF2A mutations had been identified in any cancer. First, we reported two novel somatic gain-of-function HIF2A mutations in two patients, one presenting with multiple PGLs and a second with both multiple PGLs and multiple duodenal somatostatinoma, both associated with polycythemia (Reference 2). Both mutations showed increased HIF2alpha activity and protein half-life. While germline mutations of HIF2α regulators, including VHL, EGLN1, SDHB, SDHC, and SDHD, had been reported in PHEOs/PGLs, this was the first report of a somatic gain-of-function mutation in HIF2α. Subsequently, we investigated two additional unrelated patients and found them to present with the same disease cluster (Jochmanova et al., J Natl Cancer Inst 2013;105:1270-1283). Recently, we described new ocular findings in the patients, findings that indicate the existence of a new syndrome with multiple PGLs and somatostatinomas associated with polycythemia (Pacak-Zhuang syndrome). The new syndrome results from somatic gain-of-function HIF2A mutations, which cause an upregulation of hypoxia-related genes, including that encoding erythropoietin (EPO) and genes important in cancer biology.
We investigated the genetic/pathogenetic factors associated with a new clinical entity in patients presenting with PHEO/PGL and polycythemia. Two patients without hypoxia-inducible factor 2α (HIF2A) mutations, who presented with similar clinical manifestations, were analyzed for other gene mutations, including prolyl hydroxylase (PHD) mutations. We found, for the first time, a germ-line mutation in PHD1 in one patient and a novel germ-line PHD2 mutation in a second patient. Both mutants resulted in reduced protein stability with substantial quantitative protein loss and thus compromised catalytic activities. Given the unique association of patients' polycythemia with borderline or mildly elevated erythropoietin (EPO) levels, we also performed an in vitro sensitivity assay of erythroid progenitors to EPO and for EPO receptor (EPOR) expression. The results show inappropriate hypersensitivity of erythroid progenitors to EPO in these patients, indicating increased EPOR expression/activity. In addition, the study indicates that HIF dysregulation resulting from PHD mutations plays an important role in the pathogenesis of these tumors and associated polycythemia. The PHD1 mutation appears to be a new member contributing to the genetic landscape of this novel clinical entity. Our results support the existence of a specific PHD1– and PHD2–associated PHEO/PGL–polycythemia disorder.
Imaging of various pheochromocytomas and paragangliomas
The aim of this pilot study was to determine whether metabolic tumor volume (MTV) and total lesion glycolysis (TLG) could serve as predictors of biochemical remission and pharmacotherapy-free interval in patients with metastatic PHEO/PGL. Patients with metastatic PHEOs/PGLs have a high rate of biochemical recurrence, which can be associated with increased cardiovascular morbidity. Predictors of biochemical response are needed to guide and select patients who may benefit from therapy. We calculated whole body MTV and TLG from preoperative 18F-FDG PET/CT scans and analyzed them as markers of biochemical response and pharmacotherapy-free interval. Seventeen patients underwent a total of 19 procedures, with a median follow-up time of 26.4 months. Whole body MTV of patients with biochemical recurrence (n = 13, mean 73.8 mL) was higher than those who had a biochemical response (n = 6, mean 14.7 mL). Patients with low MTV (less than 37.2 mL) had a significantly improved durable partial biochemical response, and a statistical trend for complete biochemical remission and pharmacotherapy-free interval. In eight patients with metastatic disease outside the abdomen, four had less than 35% of their disease burden outside the abdomen, and these patients had a significantly more durable partial biochemical response than did patients with greater than 35% of their disease burden outside the abdomen. Whole body MTV and TLG represents novel and valuable predictors of biochemical response for patients with metastatic PHEOs/PGLs. A larger prospective study should be performed to validate these findings.
SDHx–related PHEOs/PGLs are characterized by compromised oxidative phosphorylation and a pseudohypoxic response, which mediates an increase in aerobic glycolysis, also known as the Warburg effect. We explored the hypothesis that increased uptake of 18F-FDG in SDHx-related PHEOs/PGLs is reflective of increased glycolytic activity and correlated with expression of different proteins involved in glucose uptake and metabolism through the glycolytic pathway. We investigated twenty-seven PHEOs/PGLs collected from patients with hereditary mutations in SDHB (n = 2), SDHD (n = 3), RET (n = 5), NF-1 (neurofibromatosis type 1) (n = 1), and MAX (myc-associated factor X) (n = 1) as well as sporadic patients (n = 15). Preoperative 18F-FDG PET/CT studies were analyzed; mean and maximum standardized uptake values (SUVs) in manually drawn regions of interest were calculated. We examined the expression of proteins involved in glucose uptake (glucose transporters types 1 and 3 [GLUT-1 and -3, respectively]), phosphorylation (hexokinases 1, 2, and 3 [HK-1, -2, and -3, respectively]), glycolysis (monocarboxylate transporter type 4 [MCT-4]), and angiogenesis (vascular endothelial growth factor [VEGF], CD34) in paraffin-embedded tumor tissues using immunohistochemical staining with peroxidase-catalyzed polymerization of diaminobenzidine as a read-out. The expression was correlated with corresponding SUVs. Both maximum and mean SUVs for SDHx–related tumors were significantly higher than those for sporadic and other hereditary tumors. The expression of HK-2 and HK-3 was significantly higher in SDHx–related PHEOs/PGLs than in sporadic PHEOs/PGLs. The expression of HK-2 and VEGF was significantly higher in SDHx–related PHEOs/PGLs than in other hereditary PHEOs/PGLs. No statistical differences in the expression were observed for GLUT-1, GLUT-3, and MCT-4. The percentage anti-CD34 staining and mean vessel perimeter were significantly higher in SDHx–related PHEOs/PGLs than in sporadic tumors. Mean SUVs significantly correlated with the expression of HK-2, HK-3, VEGF, and MCT-4. The activation of aerobic glycolysis in SDHx–related PHEOs/PGLs is associated with increased 18F-FDG accumulation owing to accelerated glucose phosphorylation by hexokinases rather than increased expression of glucose transporters.
Therapeutic aspects of pheochromocytoma and paraganglioma
Multi-modality therapy is used in treating malignant PHEO/PGL; however, few data exist on the role of external beam radiation therapy (EBRT). In a retrospective review, we assessed response to EBRT in malignant PHEOs or PGLs. We studied records of patients treated at the NIH who received EBRT between 1990 and 2012. Patients were assessed for symptomatic control, biochemical response, local and distant control by response evaluation criteria in solid tumors or stable disease on imaging reports, toxicity by radiation therapy oncology group (RTOG) criteria, and survival. There were 47 patients treated who received EBRT to lesions either of the abdomen (n = 3), central nervous system (n = 4), or bone (n = 40). Lesions were treated with 3D conformal EBRT to a mean dose of 31.8 Gy in 3.3 Gy fractions, or fractionated stereotactic radiosurgery to 21.9 Gy in 13.6 Gy fractions. Patients experienced acute (n = 15) and late (n = 2) RTOG toxicities. Symptomatic control was achieved in 81.1% of lesions. Stable radiographic response was achieved in 86.7% of lesions with progression in 13%. Distant progression was observed overall in 75% of patients and average survival was 52.4 months. In conclusion, malignant PHEO/PGL often do not respond well to current systemic therapies. In these cases, EBRT can be considered in patients with symptomatic, localized disease progression.
To date, malignant PHEOs/PGLs cannot be effectively cured, and thus novel treatment strategies are urgently needed. Lovastatin has been shown to effectively induce apoptosis in mouse PHEO cells (MPC) and the more aggressive mouse tumor tissue–derived cells (MTT), which was accompanied by reduced phosphorylation of mitogen-activated kinase (MAPK) pathway players. The MAPK pathway plays a role in numerous aggressive tumors and has been associated with a subgroup of PHEOs/PGLs, including K-RAS–, RET–, and NF1–mutated tumors. Our aim was to establish whether MAPK signaling plays a role in aggressive, succinate dehydrogenase (SDH) B mutation–derived PHEOs/PGLs. Expression profiling and western blot analysis indicated that specific aspects of MAPK signaling are active in SDHB PHEOs/PGLs, suggesting that inhibition by statin treatment could be beneficial. Moreover, we aimed to assess whether the anti-proliferative effect of lovastatin on MPC and MTT differed from that exerted by fluvastatin, simvastatin, atorvastatin, pravastatin, or rosuvastatin. Simvastatin and fluvastatin reduced cell proliferation most effectively, and the more aggressive MTT cells appeared more sensitive in this respect. Inhibition of MAPK1 and/or MAPK3 phosphorylation following treatment with fluvastatin, simvastatin, or lovastatin was confirmed by western blot. Increased levels of CASP-3 and PARP cleavage confirmed induction of apoptosis following the treatment. At a concentration low enough not to affect cell proliferation, spontaneous migration of MPC and MTT was significantly inhibited within 24 hours of treatment. In conclusion, lipophilic statins may present a promising therapeutic option for treatment of aggressive human PGLs by inducing apoptosis and inhibiting tumor spread.
Currently, there are no reliably effective therapeutic options for metastatic PHEO/PGL. Moreover, there are no therapies that may prevent the onset or progression of tumors in patients with SDHB, which are associated with very aggressive tumors. Therefore, we tested the approved and well-tolerated drugs lovastatin and 13-cis-retinoic acid (13cRA) in vitro in an aggressive pheochromocytoma (PCC) mouse cell line, mouse tumor tissue-derived (MTT) cells, and in vivo in a PCC allograft nude mouse model, in therapeutically relevant doses. Treatment was started 24 hours before tumor cell injection and continued for 30 more days. We measured tumor sizes from the outside by caliper and sizes of viable tumor mass by bioluminescence imaging. Lovastatin showed antiproliferative effects in vitro and led to significantly smaller tumor sizes in vivo than with vehicle treatment. 13cRA promoted tumor cell growth in vitro and led to significantly larger viable tumor mass and significantly faster increase of viable tumor mass in vivo over time than with vehicle, lovastatin, or combination treatment. However, when combined with lovastatin, 13cRA enhanced the antiproliferative effect of lovastatin in vivo. Combination-treated mice showed the slowest tumor growth of all groups with significantly slower tumor growth than in vehicle-treated mice and significantly smaller tumor sizes. Moreover, the combination-treated group displayed the smallest size of viable tumor mass and the slowest increase in viable tumor mass over time of all groups, with a significant difference compared with the vehicle- and 13cRA–treated group. The combination-treated tumors showed the highest extent of necrosis, lowest median microvessel density, and highest expression of α-smooth muscle actin. The combination of high microvessel density and low α-smooth muscle actin is a predictor of poor prognosis in other tumor entities. Therefore, the drug combination may be a well-tolerated novel therapeutic or preventive option for malignant PHEO/PGL.
Animal model of pheochromocytoma and cell culture studies
The lack of sensitive animal models of PHEO has hindered the study of this tumor and in vivo evaluation of antitumor agents. Previously, we generated two sensitive luciferase models using bioluminescent PHEO cells: an experimental metastasis model to monitor tumor spreading; and a subcutaneous model to monitor tumor growth and spontaneous metastasis. The models offer a platform for sensitive, non-invasive, and real-time monitoring of PHEO primary growth and metastatic burden to follow the course of tumor progression and for testing relevant antitumor treatments in metastatic PHEO. Currently, we are testing several new drugs on this animal model.
Publications
- Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med 2012; 367:922-930.
- Pacak K, Jochmanova I, Prodanov T, Yang C, Merino MJ, Fojo T, Prchal JT, Tischler AS, Lechan RM, Zhuang Z. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol 2013; 31:1690-1698.
- Schovanek J, Martucci V, Wesley R, Fojo T, Del Rivero J, Huynh T, Adams K, Kebebew E, Frysak Z, Stratakis CA, Pacak K. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer 2014; 14:523.
- Fliedner SM, Engel T, Lendvai NK, Shankavaram U, Nölting S, Wesley R, Elkahloun AG, Ungefroren H, Oldoerp A, Lampert G, Lehnert H, Timmers H, Pacak K. Anti-cancer potential of MAPK pathway inhibition in paragangliomas-effect of different statins on mouse pheochromocytoma cells. PloS One 2014; 9:e97712.
- Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P, Taïeb D, Kebebew E, Lehnert H, Fojo AT, Pacak K. Superiority of [68Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin Cancer Res 2015; 21:3888-3895.
Collaborators
- Clara C. Chen, MD, Nuclear Medicine Department, Clinical Center, NIH, Bethesda, MD
- Graeme Eisenhofer, PhD, Universität Dresden, Dresden, Germany
- Abdel G. Elkahloun, PhD, Genome Technology Branch, NHGRI, NIH, Bethesda, MD
- Tito Fojo, MD, PhD, Medical Oncology Branch, NCI, Bethesda, MD
- Electron Kebebew, MD, Surgery Branch, NCI, Bethesda, MD
- Ron Lechan, MD, PhD, Tufts Medical Center, Boston, MA
- Jacques Lenders, MD, Radboud Universiteit, Nijmegen, The Netherlands
- W. Marston Linehan, MD, Urologic Oncology Branch, NCI, Bethesda, MD
- Alexander Ling, MD, Radiology Department, Clinical Center, NIH, Bethesda, MD
- Lani Mercado-Asis, MD, PhD, University of Santo Tomas, Manila, Philippines
- Maria J. Merino, MD, Pathology Department, NCI, Bethesda, MD
- Corina Millo, MD, PET Department, Clinical Center, NIH, Bethesda, MD
- Margarita Raygada, PhD, Program in Reproductive and Adult Endocrinology, NICHD, Bethesda, MD
- James C. Reynolds, MD, Nuclear Medicine Department, Clinical Center, NIH, Bethesda, MD
- Constantine A. Stratakis, MD, D(med)Sci, Program in Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- Henri Timmers, MD, PhD, Radboud Universiteit, Nijmegen, The Netherlands
- Arthur S. Tischler, MD, PhD, New England Medical Center, Boston, MA
- Robert A. Wesley, PhD, Clinical Epidemiology and Biostatistics Service, Clinical Center, NIH, Bethesda, MD
- Zhengping Zhuang, MD, PhD, Surgical Neurology Branch, NINDS, Bethesda, MD
Contact
For more information, email karel@mail.nih.gov or visit http://pheopara.nichd.nih.gov.


