Assembly and Function of Chromatic Circuits in Drosophila

- Chi-Hon Lee, MD, PhD, Head, Section on Neuronal Connectivity
- Chun-Yuan Ting, PhD, Staff Scientist
- Yang Li, PhD, Postdoctoral Fellow
- Jiangnan Luo, PhD, Postdoctoral Fellow
- Pushpanathan Muthuirulan, PhD, Postdoctoral Fellow
- Bo Shi, MSc, Graduate Student
- Moyi Li, BA, Biological Laboratory Technician
Using the Drosophila visual system as a model, we study how neurons form complex yet stereotyped synaptic connections during development and how the assembled neural circuits extract visual attributes, such as color and motion, to guide animal behaviors. To study visual circuit functions, we combine structural and functional approaches to map visual circuits. By targeted manipulation of neuronal activity, we identified specific neurons that are functionally required for color-driven behaviors. Using both light- and electron-microscopy (EM) studies, we mapped their synaptic circuits. For circuit development, we focus on the formation of synaptic connections between the chromatic photoreceptors and their synaptic partners in the medulla neuropil. We used high-resolution imaging techniques and genetic manipulations to delineate the molecular mechanisms that control dendritic patterning and synaptic specificity of the medulla neurons.
Mapping color-vision circuits
Click image to enlarge.
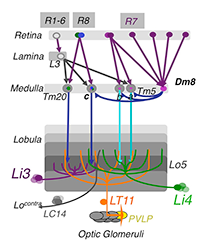
Figure 1. The chromatic circuit in Drosophila
Fly vision is mediated by three types of photoreceptors, R1–6, R7, and R8, each responding to a specific spectrum of light and connecting to different synaptic partners in the lamina and medulla neuropils. The chromatic photoreceptors R7 and R8 provide inputs for the amacrine neuron Dm8 and the transmedulla neurons Tm5a/b/c and Tm20. The transmedulla neurons transmit visual signals to four types of lobular neurons, LT11, LC14, Li3, and Li4, in the higher visual center.
Most visual animals utilize spectral information in two major ways: color vision, which differentiates spectral compositions independent of brightness, provides animals, from insects to primates, with great power for object recognition and memory registration and retrieval; innate spectral preference, on the other hand, is intensity-dependent and reflects individual species' specific ecological needs. Using a combination of genetic, histological, electrophysiological, and behavioral approaches, we study how visual circuits process chromatic information to guide behaviors in Drosophila. The receptor mechanism for color vision and spectral preference is well understood but not post-receptor processing of chromatic information. Our strategy is to: (1) identify key neuronal types and to map their synaptic connections; (2) examine the functional requirement of identified neurons for color vision and spectral preference behaviors; and (3) determine the neurotransmitter and receptor systems utilized in synaptic circuits.
Using molecular genetics and histology, we mapped the synaptic circuits of the chromatic photoreceptors R7 and R8 and their synaptic target neurons, Tm and Dm neurons, in the peripheral visual system. The medulla projection (Tm) neurons, analogous to vertebrate retinal ganglion cells, relay photoreceptor information to higher visual centers while the distal medulla (Dm) neurons connect photoreceptors to Tm neurons. Using a modified GRASP (GFP reconstitution across synaptic partners) method, we characterized the synaptic connections between photoreceptors and the Tm/Dm neurons. We found that the chromatic photoreceptors R7 (UV-sensing) and R8 (blue/green-sensing) provide inputs to a subset of first-order interneurons. The first-order interneurons Tm9, Tm20, and Tm5c receive direct synaptic inputs from R8s while Tm5a/b receive direct synaptic inputs from R7s. The Tm neurons relay spectral information from the medulla to the lobula, the deep visual processing center. In addition to the direct pathways from photoreceptors to Tm neurons, the amacrine neuron Dm8 receives inputs from multiple R7s and provides input for Tm5a/b/c.
To assign neurons to innate spectral preference, we systematically inactivated various first-order interneurons and examined the behavioral consequences. We had previously found that the amacrine Dm8 neurons, which receive UV–sensing R7 photoreceptor inputs, are both required and sufficient for animals' innate spectral preference to UV light. We found that inactivating Tm5c, one of Dm8’s synaptic targets, abolished UV preference, indicating that Tm5c is the key downstream targets for spectral preference. Using the single-cell transcript profiling technique, we found that both Dm8 and Tm5c express the vesicular glutamate transporter (VGlut), suggesting that they are glutamatergic. RNAi-knockdown of VGlut in Dm8 or Tm5c significantly reduced UV preference, suggesting the critical functions of the glutamatergic output of Dm8 and Tm5c. We further found that Tm5c expresses four kainite-type glutamate receptors and that RNAi–knockdown of these receptors, which prevents Tm5c from receiving Dm8 inputs, significantly reduced UV preference. Thus, the R7s→Dm8→Tm5c connections constitute a hard-wired glutamatergic circuit for detecting dim UV light.
Click image to enlarge.
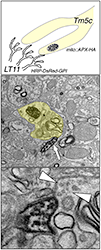
Figure 2. "Two-color" double-labeling EM
To map the synaptic circuits in complex neuropil, we developed a double-labeling method that allows the visualization of both pre- and post-synaptic partners at the ultrastructural level. We tag the presynaptic (Tm5c) mitochondria with an engineered ascorbate peroxidase and the dendritic membrane (LT11) with a membrane-tethered horseradish peroxidase. We are thus able to identify both presynaptic and postsynaptic partners in a single EM preparation.
To identify color-vision circuits, we developed a novel aversive operant conditioning assay for intensity-independent color discrimination. We conditioned single flies to discriminate between equiluminant blue or green stimuli. We found that wild-type flies can be trained to avoid either blue or green while mutants lacking functional R7 and R8 photoreceptors cannot, indicating that color entrainment requires the function of the narrow-spectrum photoreceptors R7 and/or R8. Inactivating all four types of first-order interneurons, Tm5a/b/c and Tm20, abolishes color learning. However, inactivating different subsets of these neurons is insufficient to block color learning, suggesting that true color vision is mediated by parallel pathways with redundant functions. The apparent redundancy in learned color discrimination sharply contrasts with innate spectral preference, which is dominated by a single pathway, R7→Dm8→Tm5c.
To determine how color information is processed in the higher visual center, we set out to identify the lobula neurons that receive direct synaptic inputs from the chromatic Tm neurons Tm5a/b/c and Tm20. We first collected and characterized available Gal4 lines for lobula expression. Second, we used our modified GRASP method to examine potential contacts between chromatic Tm neurons and the dendrites of candidate lobula neurons. We identified four types of lobula neuron that form synaptic contacts with chromatic Tm neurons: two novel lobula-intrinsic neurons, Li3 and Li4, and two lobula projection neurons, LT11 and LC14. Each LT11 elaborates a large dendritic tree to cover all the Lo4–6 lobula layers and projects an axon to optic glomeruli in the central brain. Each Li4 extends dendrites to cover about 60% of the Lo5 lobula layer. Using the single-cell GRASP method we had developed, we further characterized the synaptic connections at single-cell resolution and found that both Li4 and LT11 neurons receive inputs from all four chromatic Tm neurons. Our anatomical study suggests that the first relay in the lobula involves both spatial and chromatic integration.
Dendritic development of Drosophila optic lobe neurons
Click image to enlarge.
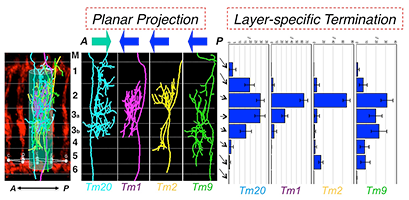
Figure 3. Type-specific dendritic attributes
By registering dendritic trees to a standard array structure, we identified two key dendritic attributes of medulla neurons: planar projections and layer-specific termination. Planar projections are the directions (anterior or posterior) of dendritic segments projecting from the axons to the medulla columns they innervate. Layer-specific termination refers to the locations of the dendritic terminals in specific medulla layers (M1–M6). The two attributes define medulla neuronal types.
Assembling a functional neural circuit requires guiding axons and dendrites to appropriate regions to form synaptic connections during development. Neurodevelopmental disorders, such as Fragile X syndrome and Rett syndrome, have distinct defects in dendritic morphologies, including alteration of branching patterns, loss of branches, and changes in spine shapes and numbers. Many mental diseases, such as mental retardation, schizophrenia, and autism spectrum disorder, have hidden developmental origins, and their neurological and cognitive deficits, characteristic of neural miscommunication, are likely caused by dendritic defects. It is unclear however, how genetic defects cause dendritic patterning defects, resulting in erroneous connections and functional deficit.
We use the Drosophila visual neurons as a model to study dendrite development in the central nervous system. Like the vertebrate cortex and retina, the Drosophila optic lobe is organized in columns and layers, suggesting that fly visual neurons and vertebrate cortex neurons face similar challenges in routing their dendrites to specific layers and columns during development. In addition, the Drosophila visual system has several unique advantages: (1) the medulla neurons extend dendritic arbors in a lattice-like structure, facilitating morphometric analysis; (2) the synaptic partnership is known; (3) genetic tools for labeling specific classes of medulla neurons and determining their connectivity are available; (4) sensitive behavioral assays are available for quantifying functional deficits. To analyze the dendritic patterns of the medulla neurons, we developed several new techniques: a dual-imaging technique for high-resolution imaging; a registration technique to standardize and to compare dendritic patterns; and a modified GRASP method for visualizing bona fide synaptic connections at the light-microscopic level.
Using these techniques, we first analyzed the dendritic morphologies of four types of medulla neurons, Tm1, Tm2, Tm9 and Tm20. We identified four dendritic attributes: (1) over 80% of dendritic branches arise from one or two primary branching nodes in the medulla M2–3 layers; (2) dendrites project from the primary branching points in an either anterior or posterior direction (planar directions) in a type-specific fashion; (3) dendrites terminate in specific layers (layer-specific distribution) in a type-specific fashion; (4) the dendrites of four types of Tm neurons are largely confined to single medulla columns. By cluster analyses, using either PCA (principle component analysis) or information theory–based t-SNE (t-distributed stochastic neighboring embedding) algorithm, we found that layer-specific distribution of dendritic terminals and planar projection directions are the most important type-specific attributes and that they are sufficient to differentiate the Tm neurons. In sharp contrast, standard morphometric parameters, such as branch numbers and bifurcation topologies, are similar among these Tm neurons, and these parameters are incapable of categorizing Tm neurons' dendritic patterns. Furthermore, the other two attributes, layer-specific location of primary branching nodes and the dendritic field sizes, differentiate Tm1/2/9/20 from other Tm neurons and Dm8 neurons.
To determine the molecular mechanisms controlling dendritic patterning during development, we screened loss-of-function mutations for morphological defects in Tm20 and Dm8 dendrites. We identified mutations that specifically affect distinct aspects of dendritic patterning, including layer-specific location of primary branching nodes, planar projection directions, and dendritic field sizes. We focused on the components of the TGF-beta/Activin signaling pathway, which specifically affect the size of the dendritic fields of Tm20 and Dm8. Single-cell mosaic analyses revealed that mutant Tm20 lacking Activin signaling components, such as the receptor Baboon and the downstream transcription factor Smad2, elaborated an expanded dendritic tree spanning several medulla columns. Morphometric analyses based on a Kaplan-Meier non-parametric estimator further showed that baboon and smad2 mutations significantly reduced dendritic termination frequency. Using a modified GRASP method, we found that the expanded dendritic tree of mutant Tm20 forms aberrant synaptic contacts with several neighboring R8 photoreceptors. In contrast, wild-type Tm20 forms synaptic connections with single R8 photoreceptors in its cognate column. Thus, the loss of Activin signaling in Tm20 neurons not only affects the size of their dendritic fields but also results in the formation of incorrect synaptic connections. Similarly, removing Baboon or Smad2 in Dm8 neurons resulted in expanded dendritic fields as compared with the wild-type. Conversely, overexpressing a dominant active form of Baboon in developing Dm8 resulted in reduced dendritic field sizes. Thus, Activin signaling negatively regulates the sizes of dendritic fields of Tm20 and Dm8.
To search for the source of TGF-beta ligands for Tm20 and Dm8, we carried out in situ hybridization and RT-PCR for all four potential ligands, Activin, Dawdle, Myoglianin, and Maverick, and found Activin expression in developing R7 and R8 photoreceptor neurons. RNAi–mediated knockdown of Activin in R7s and R8s caused abnormal expansion of dendritic fields of Dm8 and Tm20, respectively. The results indicate that photoreceptors R7 and R8 provide Activin specifically for their respective synaptic targets, Dm8 and Tm20. Interestingly, while the R7 and R8 growth cones were only a few micrometers apart, the Activin they provide is incapable of replacing that of the other, suggesting that Activin acts at a very short range. In summary, we found that photoreceptor-derived Activin controls the dendritic development of their respective synaptic target neurons, suggesting that anterograde Activin signaling coordinates afferent target development.
Publications
- Ting C-Y, McQueen PG, Pandya N, Lin T-Y, Yang M, Reddy OV, O’Connor MB, McAuliffe M, Lee C-H. Photoreceptor-derived activin promotes dendritic termination and restricts the receptive fields of first-order interneurons in Drosophila. Neuron 2014; 81:830-846.
- Karuppudurai T, Lin T-Y, Ting C-Y, Pursley R, Melnattur KV, Diao F, White BH, Macpherson LJ, Gallio M, Pohida T, Lee C-H. A hard-wired glutamatergic circuit pools and relays UV signals to mediate spectral preference in Drosophila. Neuron 2014 81:603-615.
- Melnattur KV, Pursley R, Lin T-Y, Ting C-Y, Smith PD, Pohida T, Lee C-H. Multiple redundant medulla projection neurons mediate color vision in Drosophila. J Neurogenet 2014; 28:374-88.
- Lin T-Y, Luo J, Shinomiya K, Ting C-Y, Lu Z, Meinertzhagen IA, Lee C-H. Mapping chromatic pathways in the Drosophila visual system. J Comp Neurol 2016; 1;524(2):213-27.
- Macpherson LJ, Kearney PJ, Zaharieva EE, Lin T-Y, Turan Z, Lee C-H, Gallio M. Dynamic, multiple-color labeling of neural connections by trans-synaptic fluorescence complementation. Nat Commun 2015; 6:10024.
Collaborators
- Dion Dickman, PhD, University of Southern California, Los Angeles, CA
- Marco Gallio, PhD, Northwestern University, Evanston, IL
- Mary Lilly, PhD, Cell Biology and Metabolism Program, NICHD, Bethesda, MD
- Mark Mayer, PhD, Laboratory of Cellular and Molecular Neurophysiology, NICHD, Bethesda, MD
- Matthew McAuliffe, PhD, Division of Biomedical Imaging Research Services Section, CIT, NIH, Bethesda, MD
- Philip McQueen, PhD, Mathematical and Statistical Computing Laboratory, CIT, NIH, Bethesda, MD
- Ian Meinertzhagen, PhD, DSc, Dalhousie University, Halifax, Canada
- Kate O’Connor-Giles, PhD, University of Wisconsin, Madison, WI
- Nishith Pandya, BA, Division of Biomedical Imaging Research Services Section, CIT, NIH, Bethesda, MD
- Thomas Pohida, MSEE, Division of Computational Bioscience, CIT, NIH, Bethesda, MD
- Randy Pursley, MSEE, Division of Computational Bioscience, CIT, NIH, Bethesda, MD
- Mihaela Serpe, PhD, Program in Cellular Regulation and Metabolism, NICHD, Bethesda, MD
- Mark Stopfer, PhD, Program in Developmental Neuroscience, NICHD, Bethesda, MD
- Benjamin White, PhD, Laboratory of Molecular Biology, NIMH, Bethesda, MD


