Hippocampal Interneurons and Their Role in the Control of Network Excitability

- Chris J. McBain, PhD, Head, Section on Cellular and Synaptic Physiology
- Kenneth Pelkey, PhD, Staff Scientist
- Ramesh Chittajulla, PhD, Senior Research Fellow
- Gulcan Akgul, PhD, Postdoctoral Fellow
- Michael Craig, PhD, Postdoctoral Fellow
- Robert Mitchell, PhD, Postdoctoral Fellow
- Jason Wester, PhD, Postdoctoral Fellow
- Megan Wyeth, PhD, Postdoctoral Fellow
- Geoff Vargish, BS, Graduate Student
- Xiaoqing Yuan, MSc, Biologist
- Steven Hunt, Biologist
Cortical and hippocampal local-circuit GABAergic inhibitory interneurons are 'tailor-made' to control Na+- and Ca2+-dependent action potential generation, regulate synaptic transmission and plasticity, and pace large-scale synchronous oscillatory activity. The axons of this diverse cell population make local, usually short-range projections (some subpopulations project their axons over considerable distances) and release the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) onto a variety of targets. A mounting appreciation of the roles played by interneurons in several mental health conditions such as epilepsy, stroke, Alzheimer's disease, and schizophrenia have placed this important cell type center stage in cortical circuit research. Our main objective is to understand the developmental programs that regulate their integration into cortical circuits and how both ionic and synaptic mechanisms regulate the activity of cortical neurons at the level of small, well defined networks. To this end, we use a variety of electrophysiological, immunohistochemical, molecular, and genetic approaches in both wild-type and transgenic animals. Over the past few years, we have continued our study of the differential mechanisms of glutamatergic and GABAergic synaptic transmission and plasticity within the hippocampal formation and the modulation of voltage- and ligand-gated channels expressed in inhibitory neurons. We also incorporate genetic approaches to unravel the embryogenesis and development of hippocampal interneurons and the circuits in which they are embedded. We are particularly interested in discovering the rules that dictate co-ordinated protein expression in nascent interneuron subpopulations as they migrate and integrate into the developing cortical circuit.
Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons.
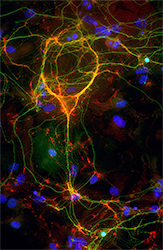
Click image to enlarge.
GluA4–decorated parvalbumin-containing interneuron
A parvalbumin (green)-containing inhibitory interneuron stained for the glutamate receptor subunit GluA4 (red)
Circuit computation requires precision in the timing, extent, and synchrony of principal cell (PC) firing that is largely enforced by parvalbumin-expressing, fast-spiking interneurons (PVFSIs). To reliably coordinate network activity, PVFSIs exhibit specialized synaptic and membrane properties that promote efficient afferent recruitment such as expression of high-conductance, rapidly gating AMPA receptors (AMPARs) that contain the GluA4 subunit. We found that PVFSIs upregulate GluA4 during the second postnatal week, coincident with increases in the AMPAR–clustering proteins NPTX2 and NPTXR. Moreover, GluA4 is dramatically reduced in NPTX2−/−/NPTXR−/− mice, with consequent reductions in PVFSI AMPAR function. Early postnatal NPTX2−/−/NPTXR−/− mice exhibit delayed circuit maturation with a prolonged critical period permissive for giant depolarizing potentials. Juvenile NPTX2−/−/NPTXR−/− mice display reduced feedforward inhibition, yielding a circuit deficient in rhythmogenesis and prone to epileptic discharges. Our findings demonstrate an essential role for NPTX proteins in controlling network dynamics, highlighting potential therapeutic targets for disorders with inhibition/excitation imbalances such as schizophrenia.
Fast gamma oscillations are generated intrinsically in CA1 without the involvement of fast-spiking basket cells
Information processing in neuronal networks relies on the precise synchronization of ensembles of neurons, coordinated by the diverse family of inhibitory interneurons. Cortical interneurons can be usefully parsed by embryonic origin, with the vast majority arising from either the caudal or medial ganglionic eminences (CGE and MGE). We examined the activity of hippocampal interneurons during gamma oscillations in the mouse CA1 hippocampal region, using an in vitro model in which brief epochs of rhythmic activity were evoked by local application of kainate (KA). We found that this CA1 KA–evoked gamma oscillation was faster than that in the CA3 region and, crucially, did not appear to require the involvement of fast-spiking basket cells. In contrast to CA3, we also found that optogenetic inhibition of pyramidal cells in CA1 did not significantly affect the power of the oscillation, suggesting that excitation may not be essential for gamma genesis in this region. We found that MGE–derived interneurons were generally more active than CGE interneurons during CA1 gamma oscillations, although a group of CGE–derived interneurons, putative trilaminar cells, were strongly phase-locked with gamma oscillations and, together with MGE–derived axo-axonic and bistratified cells, provide attractive candidates for being the driver of this locally generated, predominantly interneuron-driven model of gamma oscillations.
Neto auxiliary protein interactions regulate kainate and NMDA receptor subunit localization at mossy fiber-CA3 pyramidal cell synapses.
Neto1 and Neto2 auxiliary subunits co-assemble with NMDA receptors (NMDARs) and kainate receptors (KARs) to modulate the receptors' function. In the hippocampus, Neto1 enhances the amplitude and prolongs the kinetics of KAR–mediated currents at mossy fiber (MF)-CA3 pyramidal cell synapses. However, whether Neto1 trafficks KARs to synapses or simply alters channel properties is unresolved. Therefore, we performed post-embedding electron microscopy to investigate the localization of GluK2/3 subunits at MF-CA3 synapses in Neto-null mice. Postsynaptic GluK2/3 immunogold labeling was substantially reduced in Neto-null mice compared with wild types. Moreover, spontaneous KAR–mediated synaptic currents and metabotropic KAR signaling were absent from CA3 pyramidal cells of Neto-null mice. A similar loss of ionotropic and metabotropic KAR function was observed in Neto1–, but not Neto2–, single knock-out mice, specifically implicating Neto1 in regulating CA3 pyramidal cell KAR localization and function. Additional controversy pertains to the role of Neto proteins in modulating synaptic NMDARs. While immunogold labeling for GluN2A at MF-CA3 synapses was comparable between wild-type and Neto-null mice, labeling for postsynaptic GluN2B was robustly increased in such mice. Accordingly, NMDAR–mediated currents at MF-CA3 synapses exhibited increased sensitivity to a GluN2B–selective antagonist in Neto1 knockouts compared with wild types. Thus, despite preservation of the overall MF-CA3 synaptic NMDAR–mediated current, loss of Neto1 alters the NMDAR subunit composition. The results confirm that Neto protein interactions regulate synaptic localization of KAR and NMDAR subunits at MF-CA3 synapses, with implications for both ionotropic and metabotropic glutamatergic recruitment of the CA3 network.
Additional Funding
- Megan Wyeth was funded by a PRAT Fellowship.
- Jason Wester was funded by an NINDS Intramural NRSA award
Publications
- Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci 2013; 16:1032-1041.
- Wyeth MS, Pelkey KA, Petralia RS, Salter MW, McInnes RR, McBain CJ. Neto auxiliary protein interactions regulate kainate and NMDA receptor subunit localization at mossy fiber-CA3 pyramidal cell synapses. J Neurosci 2014; 34:622-628.
- Pelkey KA, Barksdale E, Craig MT, Yuan X-Q, Sukumaran M, Vargish GA, Mitchell RM, Wyeth MS, Petralia RS, Chittajallu R, Karlsson R-M, Cameron HA, Murata Y, Colonnese MT, Worley PF, McBain CJ. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 2015; 85:1257-1272.
- Craig MT, McBain CJ. Fast gamma oscillations are generated intrinsically in CA1 without the involvement of fast-spiking basket cells. J Neurosci 2015; 35:3616-3624.
Collaborators
- Heather Cameron, PhD, Mood and Anxiety Disorders Program, NIMH, Bethesda, MD
- Matthew Colonnese, PhD, The George Washington University School of Medicine & Health Sciences, Washington, DC
- Roderick McInnes, PhD, Lady Davis Research Institute, McGill University, Toronto, Canada
- Michael Salter, PhD, Centre for the Study of Pain, Hospital for Sick Children, Toronto, Canada
- Paul Worley, PhD, The Johns Hopkins University, Baltimore, MD
Contact
For more information, email mcbainc@mail.nih.gov or visit http://neuroscience.nih.gov/Lab.asp?Org_ID=124.


