Molecular Biology, Regulation, and Biochemistry of UDP–Glucuronosyltransferase Isozymes

- Ida S. Owens, PhD, Head, Section on Genetic Disorders of Drug Metabolism
- Nikhil K. Basu, PhD, Staff Scientist
- Amit Raychoudhuri, PhD, Visiting Fellow
- Mousumi Basu, BS, Special Volunteer
UDP–glucuronosyltransferase (UGT) isozymes–distributed primarily in liver, kidney, the gastrointestinal tract, and steroid-responsive tissues–are known to carry out the essential function of converting innumerable structurally diverse lipophilic endogenous substrates, such as neurotoxic bilirubin, catechol estrogens, and dihydrotestosterone, and dietary aromatic-like therapeutics to water-soluble excretable glucuronides. Most importantly, environmental pro-carcinogens and contaminants derived from pyrolysates are converted to avoid chemical toxicities. Our studies demonstrated that each UGT isozyme so far examined requires on-going regulated phosphate signaling, which enables an active site to convert an unspecified number of substrates. Recently, further studies showed that the human prostate luminal-cell UGT-2B15 and basal-cell UGT-2B17, which are 97% identical, have an additional Src or Src/PKCε–partnership phosphorylation site, respectively, at position 98–100. We found that the two isozymes exhibit opposite behavior when their Src sites are compromised: UGT-2B15 becomes polyubiquitinated, thus exhibiting a pro-apoptotic effect, while the activity of UGT-2B17 is elevated by 50%. Our studies will thus continue to detail and understand the specific reactions involved in human prostate–luminal cell apoptosis and de-ubiquitination. In collaboration with ongoing research within the NCI, NIDCR, and with researchers at the University of Maryland, we will also carry out basic studies to better understand prostate cancer development.
Click image to enlarge.
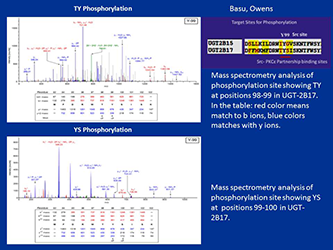
Figure 1. Mass-spectrometric analysis of TpYpSp phosphorylation of UGT-2B17
Phosphorylation of UGT-2B17 at 98–100 (TYS) residues
Prostate-distributed mouse Ugt2b34 and Ugt2b36 control estrogenic metabolites.
Click image to enlarge.
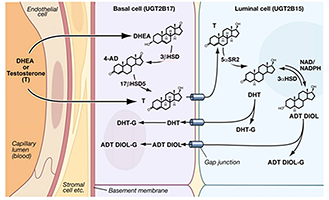
Figure 2. Predicted small-molecule movements and enzymatic reactions in prostate epithelial cells
Distribution of normal prostate steroidogenic and UGT isozymes as presented in our publication, S. K. Chakraborty et al., J Biol Chem 2012;287:24387. Based on studies cited, prostate DHT synthesis and its metabolism are summarized in the schematic. The EM shows 1:1 stratification of human prostate basal/luminal cells with intervening gap junctional structures that likely allow movement of small molecules between the two cells.
To establish an in vivo mammary-gland model that prevents depurination by 4-OH-catecholestrogens associated with the initiation of carcinogenesis, we pursued studies to identify mouse homologs of the highly effective human UGT-2B7. Using sequence analysis, we found that mouse Ugt-2b34 and Ugt-2b36 homologs avidly metabolize the test agent 4-hydroxyestrone, with Ugt-2b35 expressing trivial activity. Unlike low Km UGT-2B7 (14M), Ugt-2b34 and Ugt-2b36 metabolized 4-hydroxyestrone with 90M Km and 430M Km, respectively. Unexpectedly, the mouse isozymes are distributed primarily in male hormone–responsive tissues, whereas human UGT-2B7 is found primarily in female hormone–responsive tissues. Also, we found that Ugt-2b34 metabolizes the non-classical estrogenic DHT metabolite ADT-diol at a greater rate than DHT, which is not known to be estrogenic. Notably, UGT-2B7 does not metabolize xeno-estrogens; Ugt-2b34 and Ugt-2b36 did, however, metabolize bisphenol A (BPA) and diethylstilbestrol (DES) at superior rates. We also found, through real-time PCR–based analysis of estrogen receptor alpha (Esr1) gene knockout in mouse prostate, 50% and 63% lower Ugt2b34 mRNA and Ugt2b36 mRNA levels, respectively, than in controls. However, estrogen receptor beta (Esr2) knockout (KO) revealed a 2.7/3.3-fold increase in Ugt-2b34 mRNA and Ugt-2b36 mRNA, respectively, in prostate. Esr1 KO completely suppressed Ugt-2b34 and Ugt-2b36 mammary-gland mRNA; Esr2 KO caused a 12-fold increase in Ugt-2b34 mRNA without affecting Ugt-2b36 mRNA. Hence, according to tissue-distribution studies, it appears that male mice benefit from both Ugt isoforms, while females benefit from only one Ugt. Our findings for Ugt-2b34 and Ugt-2b36 suggest that the two mouse isozymes are intrinsically programmed to protect against a more complex environment than are human high-activity UGT-2B7 and low-activity UGT-2B4 isozymes (Raychoudhuri A et al., Biosci Rep 2015;in press).
Click image to enlarge.
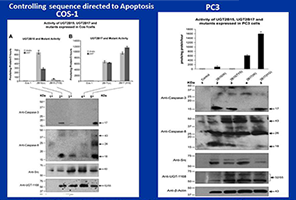
Figure 3. Controlling sequences for apoptosis
Controlling sequences for pro-apoptosis and anti-apoptosis in UGT-2B15 and UGT-2B17 expressed in COS-1 cells and the aggressive PC3 prostate cell line
Glucuronidation of the Androgens and Immunoblot Analyses for Caspases 8/3 activities. Cellular material from COS-1 (A, B) and PC3 (C) transfected cells were incubated for glucuronidaton for DHT and/or its metabolite (Andro) for 1-3h at 37º C (2A, B, C) and Western Blot analysis were carried out using antu-Caspase8, anti-Caspase3, anti-Src, anti-UGT-1168 or anti-β Actin antibodies.
Click image to enlarge.
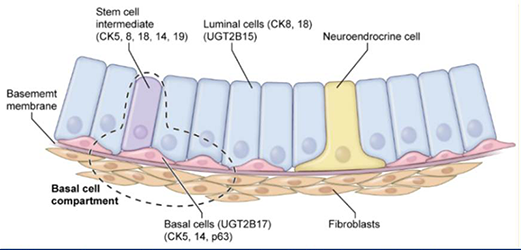
Figure 4. Diagrammatic representation of normal prostate epithelial and associated cells
Normal prostate epithelial and associated cells with UGT-2B15, UGT-2B17, and cell surface markers.
Diagrammatic representation of normal prostate epithelial and associated cells. Representations of normal human prostate luminal and basal epithelial cells surrounded by primary and intermediate-stem, neuroendocrine, normal fibroblast cells and separated by the basement membrane. A fraction of the progenitor intermediate stem cell population contains cytokeratin cell-surface markers for both (luminal) and basal [(CK8 + CK18) + CK5 + CK14 + P63] cells, respectively. Authors of these studies suggest this is evidence the two distinct prostate epithelial cells derive from a single stem cell that ultimately gives rise to two distinct differentiated epithelial cells.
Publications
- Basu NK, Kole L, Basu M, Chakraborty K, Mitra PS, Owens IS. The major chemical detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C. J Biol Chem 2008; 283:23048-23061.
- Banerjee R, Pennington MW, Garza A, Owens IS. Mapping the UDP glucuronic acid binding site in UDP-glucuronosyltransferase-1A10 by homology-based modeling: confirmation with biochemical evidence. Biochemistry 2008; 47:7385-7392.
- Mitra PS, Basu NK, Owens IS. Src supports UDP-glucuronosyltransferase-2B7 detoxification of catechol estrogens associated with breast cancer. Biochem Biophys Res Commun 2009; 382:651-656.
- Mitra PS, Basu NK, Basu M, Chakraborty S, Saha T, Owens IS. Regulated phosphorylation of a major UDP-glucuronosyltransferase isozyme by tyrosine kinases dictates endogenous substrate selection for detoxification. J Biol Chem 2011; 286:1639-1648.
- Chakraborty SK, Basu NK, Jana S, Basu M, Raychoudhuri A, Owens IS. Protein kinase Calpha and Src kinase support human prostate-distributed dihydrotestosterone-metabolizing UDP-glucuronosyltransferase 2B15 activity. J Biol Chem 2012; 287:24387-24396.
Collaborators
- Praveen Arany, BDS, MDS, MMSc, PhD, Oral and Pharyngeal Cancer Branch, NIDCR, Bethesda, MD
- James L. Gulley, MD, PhD, FACP, Genitourinary Malignancies Branch, Center for Cancer Research, NCI, Bethesda, MD
- Antony McDonagh, PhD, University of California San Francisco, San Francisco, CA
- Zhihong Nie, PhD, Maryland Nanocenter, University of Maryland, College Park, MD
- Juan Rivera, PhD, Molecular Immunology and Inflammation Branch, NIAMS, Bethesda, MD
Contact
For more information, email owens@helix.nih.gov.


