Mechanisms of Synapse Assembly, Maturation and Growth during Development

- Mihaela Serpe, PhD, Head, Unit on Cellular Communication
- Mikolaj Sulkowski, PhD, Postdoctoral Fellow
- Tae Hee Han, PhD, Visiting Fellow
- Cathy Ramos, PhD, Visiting Fellow
- Qi Wang, PhD, Visiting Fellow
- Grace Kim, BS, Postbaccalaureate Fellow
- Peter Nguyen, Biological Laboratory Technician
Synapse development is a highly orchestrated process coordinated by intercellular communication between the pre- and postsynaptic compartments and by neuronal activity itself. Our long-term goal is to elucidate the molecular mechanisms, particularly those involving cell-cell communication, that regulate formation of functional synapses during development and fine-tune them during plasticity and homeostasis. We focus on three key processes in synaptogenesis: (1) trafficking of components to the proper site, (2) organizing those components to build synaptic structures, and (3) maturation and homeostasis of the synapse to optimize its activity. We address the molecular mechanisms underlying these processes using a comprehensive set of approaches including genetics, biochemistry, molecular biology, super-resolution imaging, and electrophysiology recordings in live animals and reconstituted systems.
The Drosophila neuromuscular junction (NMJ) is a powerful genetic system in which to study mechanisms of synapse assembly and homeostasis. The fact that individual NMJs in flies can be reproducibly identified from animal to animal and are easily accessible for electrophysiological and optical analysis makes them uniquely suited for in vivo studies on synapse assembly, growth, and plasticity. Furthermore, the great richness of genetic manipulations that can be performed in Drosophila permits independent control of individual synaptic components in distinct cellular compartments. Moreover, the fly NMJ is a glutamatergic synapse similar in composition and physiology to mammalian central synapses. The Drosophila NMJ can thus be used to analyze and model defects in the structural and physiological plasticity of glutamatergic synapses, which are associated with a variety of human pathologies from learning, memory deficits, to autism. The similarity in architecture, function, and molecular machinery supports the notion that studying the assembly and development of fly glutamatergic synapses will shed light on their human counterparts.
Drosophila Neto, an obligatory auxiliary subunit for NMJ glutamate receptors
Drosophila iGluRs are heterotetrameric complexes composed of three shared subunits, GluRIIC, GluRIID, and GluRIIE and either GluRIIA (type-A receptors) or GluRIIB (type-B). The shared subunits are essential for viability—without any of them the animals are completely paralyzed and die as late embryos. We recently discovered Neto (Neuropillin and Tolloid-like), a non-channel essential component of the NMJ glutamate receptor complexes required for clustering of these receptors at synaptic sites. Through live-imaging studies we showed that Neto clusters at nascent NMJs at the time when iGluRs begin to accumulate and cluster. Our genetics and biochemical analyses indicate that Neto forms a complex with the iGluRs on the muscle membrane and that the Neto/iGluRs complexes traffic together to synaptic sites, where they form stable aggregates. By controlling the clustering and trafficking of functional iGluR complexes, Neto directly controls synapse assembly, organization and maintenance of post-synaptic densities (PSDs), and NMJ functionality.
Neto belongs to a family of highly conserved proteins sharing an ancestral role in formation and modulation of glutamatergic synapses. Neto proteins are multidomain, transmembrane proteins with two extracellular CUB [for complement C1r/C1s, UEGF, BMP-1 (bone morphogenetic protein-1)] domains followed by an LDLa (low-density lipoprotein receptor domain class A) motif, a single transmembrane pass, and highly divergent intracellular parts. CUB domains are BMP–binding, protein-interaction domains that could promote aggregation via self-association and/or extracellular interactions. CUB–containing proteins have been implicated in the formation of multi-molecular complexes, including acetylcholine receptor aggregates in C. elegans.
Using Neto as our entry point, we set out to elucidate the molecular mechanisms underlying the synaptic recruitment of iGluRs and their incorporation in stable neural clusters. Given that Neto does not have any catalytic activities, we hypothesized that Neto controls synapse development by interacting with iGluRs and/or with other proteins critical for synapse development. During the last year, we focused on several key questions in iGluR cell biology and began dissecting the individual steps of iGluR assembly, surface delivery, trafficking and stabilization at synaptic locations, function, and postsynaptic composition.
Neto prodomain removal enables iGluR stabilization and postsynaptic differentiation.
Our analysis of the Neto prodomain revealed a requirement for Neto in the iGluR stabilization at synaptic sites and initiation of postsynaptic differentiation. In Drosophila and other species that utilize L-glutamate as a neurotransmitter at their NMJs, Neto activities are restricted by an inhibitory prodomain, which must be removed by Furin-mediated proteolysis. When the prodomain cleavage is blocked, Neto is properly targeted to the muscle membrane and engages the iGluR complexes in vivo, but fails to enable the incorporation of iGluRs in stable clusters at synaptic locations. The uncleavable-Neto/iGluR complexes have very weak and diffuse synaptic distribution but retain some function, that is, these NMJ have a relatively normal response to evoked potentials. However, the NMJs lack any distinguishable postsynaptic structures, as if their postsynaptic differentiation were never initiated. Thus, synapse activity alone does not trigger postsynaptic differentiation; instead, Neto-dependent iGluR clustering initiates an active process of recruitment of PSD components and assembly of postsynaptic structures. The recruitment of PSD components is likely attributable to Neto-mediated intracellular interactions (see below). Although the molecular nature of the iGluR clustering mechanism remains to be determined, our results make it clear that the process requires the removal of the Neto prodomain, presumably to unmask its protein-interaction domains.
Neto-β orchestrates postsynaptic development at the Drosophila NMJ.
Drosophila neto encodes two isoforms, Neto-α and Neto-β, with different cytoplasmic domains, generated by alternative splicing. The cytoplasmic domains are rich in putative phosphorylation motifs and docking sites and are highly divergent among Neto proteins, probably reflecting cell/tissue-specific roles. In flies, RT-PCR indicates that both Neto isoforms are expressed in larval carcasses. However, knockdown of Neto-β in the muscle altered the NMJ morphology, whereas knockdown of Neto-α had only a mild effect on the NMJ development.
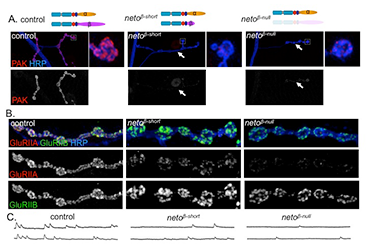
To understand the role of Neto-β in synapse development, we generated neto-β isoform–specific mutants by imprecise excision of a transposable element and isolated a neto-β genetic null allele (neto-βnull) and a truncation of the cytoplasmic domain (neto-βshort). Both neto-β mutants have locomotor defects and reduced viability. During larval stages, Neto-β–defective NMJs have reduced mini frequency and amplitudes but normal evoked potentials as a result of a compensatory increase in presynaptic release. Our analysis of the neto-β mutants reveals that Neto is directly involved in the recruitment of postsynaptic components and organization of postsynaptic structures. The neto-β synapses have reduced iGluR synaptic clusters, in particular the type-A subtypes, and lack P21–activating kinase (PAK), an important PSD component (Figure 1). PAK has been previously implicated in the stabilization of type-A receptors at postsynaptic locations and in the recruitment of other postsynaptic components, such as Dlg. Indeed, neto-β mutants have defective postsynaptic structures, including small PSDs and diminished subsynaptic reticulum. Our developmental studies indicate that Neto-β controls PAK recruitment to synaptic sites. Neto-α can rescue the lethality of netonull mutants, but fails to recruit PAK at synaptic locations, confirming the need for Neto-β in this function. Given that PAK has been implicated in stabilization of selective iGluR subtypes, Neto-β emerges as a critical player required for synapse plasticity. Thus, Neto engages in intracellular interactions that regulates the iGluR subtype composition by preferentially recruiting and/or stabilizing selective receptor subtypes.
Click image to enlarge.
Figure 1. Neto-β–mediated intracellular interactions shape postsynaptic composition.
A. Confocal images of NMJs and bouton details in larvae of indicated genotypes labeled for P21-activated kinase (PAK) (red) and horse radish peroxidase (HRP) (blue). HRP marks neuronal membranes. In the absence of Neto-β or parts of its intracellular domain, PAK fails to be recruited at synaptic locations (arrows). B. Confocal images of boutons in larvae of indicated genotypes labeled with HRP (blue) and for iGluR subunits GluRIIA (red) and GluRIIB (green). PAK is a postsynaptic component critical for the synaptic stabilization of a specific iGluR subtype, the type-A receptors. In the absence of PAK, the GluRIIA synaptic levels are drastically diminished, while the GluRIIB levels remain relatively constant. C. Traces of spontaneous miniature potentials recorded from muscle 6 of third instar larvae indicate that neto-β mutants have reduced mini amplitudes, consistent with their reduced GluRIIA/GluRIIB synaptic ratio.
Neto is required for functional iGluRs in heterologous systems.
Given that the synaptic recruitment of iGluRs is also influenced by receptor activity, Neto may regulate iGluR clustering partly by modulating the receptor function. Studies on isolated receptors are necessary to distinguish between a role for Neto on iGluR distribution and/or receptor function. The reconstitution of functional iGluRs in heterologous systems has been a powerful tool for the analysis of receptor function in other species but has not been previously achieved for the Drosophila NMJ receptors. In collaboration with Mark Mayer, we recently reported the first functional reconstitution of Drosophila NMJ iGluRs in Xenopus laevis oocytes. Using this system, we found that Neto increases the glutamate-activated currents by several orders of magnitude, but has only a modest effect on the surface delivery of the iGluRs (up to four-fold increase). Genetic studies indicate that synaptic recruitment of iGluRs requires heterotetramers. We found that only iGluR heterotetramers are efficiently delivered at the cell surface, indicating that the assembly of heterotetramers is required for surface expression.
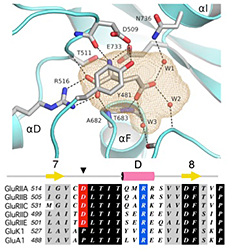
The Neto/iGluR complexes reconstituted in Xenopus oocytes recapitulate the properties of endogenous NMJ receptors: high permeability to Ca2+, block by polyamines, low affinity for glutamate, and no response to AMPA, kainate, or NMDA. To understand the structural basis for this unique ligand-binding profile, we solved the structure of the GluRIIB ligand-binding domain complex with glutamate (Figure 2). X-ray diffraction data at a resolution of 2 Å revealed a classic back-to-back dimer assembly with glutamate bound in a large cavity, similar in size to that of AMPA and kainate receptors. However, several steric clashes owing to residues conserved in the Drosophila receptors prevent the binding of AMPA and kainate and thus explain the unique ligand-binding properties observed for these receptors.
Lack of synaptic iGluRs may reflect defects in receptor assembly, surface expression, synaptic trafficking and stabilization, and/or modulation of iGluR function. Our findings pave the way to understanding the changes in receptor function before interpreting the compound phenotypes observed in vivo.
Publications
- Kim Y-J, Igiesuorobo O, Ramos CI, Bao H, Zhang B, Serpe M. Prodomain removal enables Neto to stabilize glutamate receptors at Drosophila neuromuscular junction. PLoS Genet 2015; 11:e1004988.
- Ramos CI, Igiesuorobo O, Wang Q, Serpe M. Neto-mediated intracellular interactions shape postsynaptic composition at the Drosophila neuromuscular junction. PLoS Genet 2015; 11:e1005191.
- Han TH, Poorva D, Mayer ML, Serpe M. Functional reconstitution of Drosophila NMJ glutamate receptors. Proc Natl Acad Sci USAt 2015; 112:6182-6187.
Collaborators
- Seth S. Blair, PhD, University of Wisconsin, Madison, WI
- Chi-Hon Lee, MD, PhD, Program in Cellular Regulation and Metabolism, NICHD, Bethesda, MD
- Mark Mayer, PhD, Laboratory of Cellular and Molecular Neurophysiology, NICHD, Bethesda, MD
- Bing Zhang, PhD, University of Oklahoma, Norman, OK
Contact
For more information, email serpemih@mail.nih.gov or visit http://ucc.nichd.nih.gov.