The Regulation or Disturbance of Protein/Lipid Interactions in Influenza, Malaria, Diabetes, Muscular Dystrophy, Brain Trauma, and Obesity

- Joshua Zimmerberg, MD, PhD, Head, Section on Integrative Biophysics
- Paul S. Blank, PhD, Staff Scientist
- Svetlana Glushakova, MD, PhD, Staff Scientist
- Vladimir A. Lizunov, MS, Research Fellow
- Petr Chlanda, PhD, Visiting Fellow
- Matthias Garten, PhD, Visiting Fellow
- Sourav Haldar, PhD, Visiting Fellow
- Ivonne Morales-Benavides, PhD, Visiting Fellow
- Chad McCormick, PhD, Postdoctoral Intramural Research Training Award Fellow
- Brad Busse, PhD, Postdoctoral Intramural Research Training Award Fellow
- Mariam Ghochani, MS, Graduate Student
- Ludmila Bezrukov, MS, Chemist
- Hang Waters, MS, Biologist
- Jane E. Farrington, MS, Contractor
- Elena Mekhedov, MA, Contractor
- Rea Ravin, PhD, Contractor
- Glen Humphrey, PhD, Guest Researcher
- Atsuko Kimura, PhD, Special Volunteer
Eukaryotic life must create the many shapes and sizes of the system of internal membranes and organelles that inhabit the variety of cells in nature. The membranes must remodel so that cells can secrete signaling macromolecules, express surface transporters, import macromolecular cargo, store energy, repair a damaged plasmalemma, and deal with infectious agents such as viruses and parasites. Such basic membrane mechanisms must be highly regulated and highly organized in various hierarchies in space and time to allow the organism to thrive despite environmental challenges, genetic instability, an unpredictable food supply, and physical trauma. We are using our expertise and the techniques we have perfected over the years to address several different biological problems that nevertheless share the underlying regulation or disturbance of protein/lipid interactions. Our overall goal is to determine the physico-chemical mechanisms of membrane remodeling in cells and to understand the mechanisms of cellular secretion and endocytosis at physical, biophysical, and chemical levels, including the concentration and diffusion of key vesicular components prior to and after fusion or fission.
This year, we focused on four topics: (1) the discovery that adipose cells from human subjects with varying degrees of metabolic syndrome (insulin resistance) were either refractory or sensitive to insulin in vitro, depending upon their metabolic status; (2) the formation and disintegration of a layer of protein made up of the internal scaffold of the influenza virus; (3) the colocalization of different lipids with the hemagglutinin of the influenza virus, showing no special 'raft' composition of clusters of hemagglutinin; and (4) the introduction of a new lentiviral reporter for cancer stem cells.
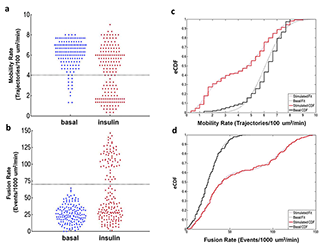
Adipose cell biopsies from insulin-resistant individuals reveal refractory fat cells.
While intercellular communication processes are frequently characterized by switch-like transitions, the endocrine system, including the adipose tissue response to insulin, has been characterized by graded responses. We found, however, that individual cells from adipose tissue biopsies are best described by a switch-like transition between the basal and insulin-stimulated states for the trafficking of the glucose transporter GLUT4. Two statistically defined populations best describe the observed cellular heterogeneity, representing the fractions of refractive and responsive adipose cells. Furthermore, subjects exhibiting high systemic insulin sensitivity indices (SI) have high fractions of responsive adipose cells in vitro, while subjects exhibiting decreasing SI have increasing fractions of refractory cells in vitro. Thus, a two-component model best describes the relationship between cellular refractory fraction and subject SI. Given that isolated cells exhibit such different response characteristics in the presence of constant culture conditions and milieu, we suggest that a physiological switching mechanism at the adipose cellular level ultimately drives systemic SI.
The matrix protein from influenza A forms and disintegrates at different pH, providing tension for membrane fusion during infection.
Influenza virus is taken up from a pH–neutral extracellular milieu into an endosome, whose contents then acidify, causing changes in the viral matrix protein (M1) that coats the inner monolayer of the viral lipid envelope. At a pH of around 6, M1 interacts with the viral ribonuclear protein (RNP) in a putative priming stage; at this stage, the interactions of the M1 scaffold coating the lipid envelope are intact. The M1 coat disintegrates as acidification continues to pH around 5 to clear a physical path for the viral genome to transit from the viral interior to cytoplasm. We investigated the physico-chemical mechanism of M1’s pH–dependent disintegration. In pH–neutral media, the adsorption of M1 protein on the lipid bilayer was electrostatic in nature and reversible. The interaction energy of M1 molecules to each other in M1 dimers was about ten-fold weaker than M1 to lipid bilayer. Acidification drives conformational changes in M1 molecules owing to changes in M1 charge, leading to alterations in their electrostatic interactions. Lowering the pH from 7.1 to 6.0 did not disturb the M1 layer; lowering it further partially desorbed M1 as a result of increased repulsion between M1 monomers still adhering to the membrane. Lipid vesicles coated with M1 demonstrated pH–dependent rupture of vesicle membrane, presumably owing to the tension generated by this repulsive force. Thus, the disruption of the vesicles coincident with M1 protein scaffold disintegration at pH 5 likely stretches the lipid membrane to the point of rupture, promoting fusion pore widening for RNP release.
It is hypothesized that the clusters of the influenza envelope protein hemagglutinin within the plasma membrane are enriched with cholesterol and sphingolipids. We directly tested this hypothesis by using high-resolution secondary ion mass spectrometry to image the distributions of antibody-labeled hemagglutinin and isotope-labeled cholesterol and sphingolipids in the plasma membranes of fibroblast cells that stably express hemagglutinin. We found that the hemagglutinin clusters were neither enriched with cholesterol nor did they colocalize with sphingolipid domains. Thus, hemagglutinin clustering and localization in the plasma membrane is controlled neither by cohesive interactions between hemagglutinin and liquid-ordered domains enriched with cholesterol and sphingolipids nor from specific binding interactions between hemagglutinin, cholesterol, and/or the majority of sphingolipid species in the plasma membrane.
Direct observation of cancer stem cells
Many tumors are hierarchically organized with a minority cell population that has stem-like properties and enhanced ability to initiate tumorigenesis and drive therapeutic relapse. Such cancer stem cells (CSCs) are typically identified by complex combinations of cell-surface markers that differ among tumor types. We developed a flexible lentiviral-based reporter system that allows direct visualization of CSCs based on functional properties. The reporter responds to the core stem-cell transcription factors OCT4 and SOX2, with further selectivity and kinetic resolution coming from use of a proteasome-targeting degron (degradation signal, i.e., sequence of amino acids determining the starting place of degradation). Cancer cells marked by this reporter have the expected properties of self-renewal, generation of heterogeneous offspring, high tumor- and metastasis-initiating activity, and resistance to chemotherapeutics. With this approach, the spatial distribution of CSCs can be assessed in settings that retain microenvironmental and structural cues, and CSC plasticity and response to therapeutics can be monitored in real time.
Additional Funding
- Jain Foundation Award
- NICHD Director’s Award (Co-Principal Investigator with Jack Yanovski)
Publications
- Batishchev OV, Shilova LA, Kachala MV, Tashkin VY, Sokolov VS, Fedorova NV, Baratova LA, Knyazev DG, Zimmerberg J, Chizmadzhev YA. pH-Dependent formation and disintegration of the influenza A virus protein scaffold to provide tension for membrane fusion. J Virol 2015; E-pub ahead of print.
- Wilson RL, Frisz JF, Klitzing HA, Zimmerberg J, Weber PK, Kraft ML. Hemagglutinin clusters in the plasma membrane are not enriched with cholesterol and sphingolipids. Biophys J 2015; 108:1652-1659.
- Lizunov VA, Stenkula KG, Blank PS, Troy A, Lee J-P, Skarulis MC, Cushman SW, Zimmerberg J. Human adipose cells in vitro are either refractory or responsive to insulin, reflecting host metabolic state. PloS One 2015; 10(3): e0119291.
- Tang B, Raviv A, Esposito D, Flanders KC, Daniel C, Nghiem BT, Garfield S, Lim L, Mannan P, Robles AI, Smith WI Jr, Zimmerberg J, Ravin R, Wakefield LM. A flexible reporter system for direct observation and isolation of cancer stem cells. Stem Cell Reports 2015; 4:155-169.
Collaborators
- Ludmila Baratova, PhD, A.N. Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, Russia
- Oleg Batishchev, PhD, A.N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russia
- Sergey Bezrukov, PhD, Program in Physical Biology, NICHD, Bethesda, MD
- Yuri Chizmadzhev, PhD, A.N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russia
- Nikki Curthoys, PhD, University of Maine, Orono, ME
- Samuel W. Cushman, PhD, Diabetes Branch, NIDDK, Bethesda, MD
- Rick M. Fairhurst, MD, PhD, Laboratory of Malaria and Vector Research, NIAID, Bethesda, MD
- Natasha Federova, MS, A.N. Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, Russia
- Vadim Frolov, PhD, Universidad del País Vasco, Bilbao, Spain
- Susan Garfield, MS, Confocal Microscopy Core Facility, Center for Cancer Research, NCI, Bethesda, MD
- Klaus Gawrisch, PhD, Laboratory of Membrane Biochemistry and Biophysics, NIAAA, Bethesda, MD
- Hugo Guerrero-Cazares, MD, The Johns Hopkins University, Baltimore, MD
- Samuel T. Hess, PhD, University of Maine, Orono, ME
- Mary Kraft, PhD, University of Illinois at Urbana-Champaign, Urbana, IL
- Jeffery Miller, MD, Molecular Medicine Branch, NIDDK, Bethesda, MD
- Gabriele Pradel, PhD, Institute of Molecular Biotechnology, RWTH Aachen University, Aachen, Germany
- Alfredo Quinones-Hinojosa, MD, The Johns Hopkins University, Baltimore, MD
- Thomas S. Reese, MD, Laboratory of Neurobiology, NINDS, Bethesda, MD
- Liudmila Shilova, MS, Moscow Institute of Physics and Technology, Moscow, Russia
- Anna Shnyrova, PhD, Universidad del País Vasco, Bilbao, Spain
- Monica Skarulis, MD, Diabetes, Endocrinology, and Obesity Branch, NIDDK, Bethesda, MD
- Valerie Sokolov, PhD, A.N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russia
- Karin G. Stenkula, PhD, Diabetes Branch, NIDDK, Bethesda, MD
- Lalage M. Wakefield, DPhil, Laboratory of Cancer Biology and Genetics, Center for Cancer Research, NCI, Bethesda, MD
- Peter K. Weber, PhD, Lawrence Livermore National Laboratory, Livermore, CA
Contact
For more information, email zimmerbj@mail.nih.gov or visit irp.nih.gov/pi/joshua-zimmerberg.