Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Melanija Tomić, PhD, Staff Scientist
- Ivana Bjelobaba, PhD, Visiting Fellow
- Marija M. Janjic, PhD, Visiting Fellow
- Milos B. Rokic, PhD, Visiting Fellow
- Jovana Tavcar, MD, Special Volunteer
- Rafael M. Previde, MD, Special Volunteer (PhD Student)
Using multidisciplinary and collaborative approaches, we investigate receptors and voltage- and ligand-gated channels, their activation by hormones and neurotransmitters, and their roles in intracellular signaling, gene expression, and hormone secretion in neuroendocrine cells. The work includes characterization of native and recombinant receptors and channels that were cloned from these cells. Currently, we are studying the calcium-mobilizing receptor-coupled gene network in non-transformed cells using RNA sequencing and qRT-PCR analysis of primary pituitary cells from developing and postpubertal mice and rats. We are also determining how the structural features of pituitary channels relate to the channels' functions and how plasma membrane receptors and the intracellular signaling milieu affect channel activity.
Cell type–specific sexual dimorphism in pituitary gene expression during maturation
In rats, the postnatal period is divided into five developmental phases based on morphological and endocrine changes: (1) neonatal [the first seven postnatal days (PND)]; (2) infantile (from PND eight to 21); (3) juvenile (from PND 22 to 30 in females and from PND 22 to 35 in males); (4) peripubertal (from PND 31 to 40 in females and PND 36 to 60 in males); and (5) adult phase (from PND 41 in females and 61 in males). Sexual differentiation of rat pituitary functions has been extensively studied at the level of hormone production and release, leading to a better understanding of developmental phases and puberty. There have also been several attempts to characterize the sex-specific transcriptional activity in pituitary glands in developing animals using various methods. However, none of the studies analyzed the differentiation of gene expression for all five secretory cell types in both sexes during development.
In a recent study, we examined the expression of major anterior pituitary genes in five secretory cell types of embryonic and developing males and females with real-time quantitative reverse transcription–PCR, using pre-designed TaqMan Gene Expression Assays, which is a sensitive and reliable method to study gene expression in both male and female pituitaries in vivo and in vitro. Corticotrophs show comparable proopiomelanocortin (Pomc) profiles in both sexes, with the highest expression occurring during the infantile period. Somatotrophs and lactotrophs also exhibit no difference in growth hormone (Gh) and prolactin (Prl) profiles during embryonic-to-juvenile age but show the amplification of Prl expression in females and Gh expression in males during peripubertal and postpubertal ages. Gonadotrophs exhibit highly synchronized luteinizing hormone beta (Lhb), follicle-stimulating hormone beta (Fshb) gonadotroph/thyrotroph-specific alpha (Cga), and gonadotropin-releasing hormone receptor (Gnrhr) gene expression in both sexes, but the peak of expression occurs during the infantile period in females and at the end of the juvenile period in males. Thyrotrophs also show different developmental thyroid-stimulating hormone beta (Tshb) profiles, which are synchronized with the expression of gonadotroph genes in males but not in females. The results indicate the lack of influence of sex on Pomc expression and the presence of two patterns of sexual dimorphism in the expression of other pituitary genes: a time shift in the peak expression during postnatal development, most likely reflecting the perinatal sex-specific brain differentiation, and modulation of the amplitude of expression during late development, which is secondary to the establishment of the hypothalamic-pituitary-gonadal and -thyroid axes (Reference 1).
Signaling, gene expression, and secretion in pituitary thyrotrophs
At present, there is limited information about signaling pathways, gene expression, and hormone release in pituitary thyrotrophs, which in part reflects difficulties in purifying thyrotrophs and single-cell identification. Mellon and co-workers developed TalphaT1 cells, which represent a differentiated thyrotroph cell line and which could provide a useful model for studies on Tshb expression and other cellular functions. Recently, we characterized, for the first time, calcium-signaling pathways in TalphaT1 cells. Our electrophysiological experiments revealed that the cells are excitable and fire action potentials spontaneously and in response to application of thyrotropin-releasing hormone (TRH), the native hypothalamic agonist for thyrotrophs. Spontaneous electrical activity is coupled with small amplitude fluctuations in intracellular calcium, whereas TRH stimulates both calcium mobilization from intracellular pools and calcium influx. Non-receptor–mediated depletion of the intracellular pool also leads to a prominent facilitation of calcium influx. Both receptor- and non-receptor–stimulated calcium influx are substantially attenuated but not completely abolished by inhibition of voltage-gated calcium channels, suggesting that depletion of the intracellular calcium pool in these cells provides a signal for both voltage-independent and -dependent calcium influx, the latter by facilitating the pacemaking activity. We also found that the cells express purinergic P2Y1 receptors and that their activation by extracellular ATP mimics TRH action on calcium mobilization and influx (Figure 1). We also found that the thyroid hormone triiodothyronine prolongs duration of TRH–induced calcium spikes during a 30-minute exposure. The data indicate that TalphaT1 cells are capable of responding to feed-forward TRH signaling and intrapituitary ATP signaling with acute calcium mobilization and sustained calcium influx. Amplification of TRH–induced calcium signaling by triiodothyronine further suggests the existence of a pathway for positive feedback effects of thyroid hormones, probably in a non-genomic manner (Reference 2).
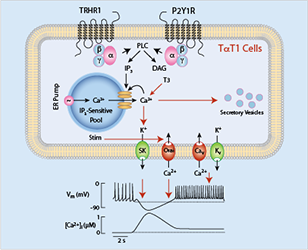
Click image to enlarge.
Figure 1. Schematic representation of calcium-signaling pathways in a thyrotroph cell line, mouse TalphaT1 cells
Cav, voltage-gated calcium channels; DAG, diacylglycerol; Kv, voltage-gated potassium channels; Nav, voltage-gated sodium channels; IP3, inositol 1,4,5-trisphosphate; Orai, store-operated calcium channels; SK, small calcium-controlled potassium channels; Stim, single-pass transmembrane protein gating Orai; T3, triiodothyronine.
In a further study on this topic, we examined the in vivo and in vitro expression pattern of three genes that are operative in the thyrotroph subpopulation from postpubertal animals: Cga, Tshb, and that encoding the thyrotropin-releasing hormone receptor (Trhr). We consistently observed the robust and sex-specific expression of Tshb and Cga during development, whereas the expression of Trhr was lower, representing 1–2% of the expression of Gapdh, the housekeeping gene, whose expression did not significantly change during development or in vitro without or with TRH. In vitro, there was a relatively rapid down-regulation of the expression of the genes in both dispersed pituitary cells and pituitary fragments; Tshb expression was most dramatically affected. In parallel with mRNA measurements, the analysis of protein content showed a significant drop in the TSHB content in cultured pituitary cells. Under continuous TRH stimulation, there was a rapid but transient stimulation of Tshb expression in pituitary fragments lasting for about 6 hours. The inhibitory effect of triiodothyronine on Tshb expression was preserved in pituitary fragments as well as in freshly dispersed and cultured pituitary cells. However, TRH applied in different concentrations periodically and continuously to freshly dispersed or cultured pituitary cells was unable to stimulate Tshb expression. Single-cell calcium measurements and secretory studies with perifused pituitary cells showed that TRH receptors were functional at least in a fraction of thyrotrophs. The loss of stimulatory effect of TRH on Tshb expression in dispersed pituitary cells indicates that additional factors contribute to TRH induction of Tshb expression in situ. We speculate that connections among thyrotrophs and/or electrical or chemical interactions between thyrotrophs and other cellular networks are critical for thyrotroph function and the proper action of TRH on gene expression (Reference 3).
Dual action of antipsychotics on lactotroph function
For decades, hyperprolactinemia (HPRL) has been recognized as a common side effect of antipsychotic medications used in the treatment of patients with schizophrenia. Moreover, HPRL has clinical consequences on physical health in both short- and long-term treatments in youth and adults alike. Among atypical antipsychotics, risperidone (RIS), a dopamine (DA) D2 receptor higher affinity full antagonist, poses the greatest risk for marked and sustained HPRL. In fact, paliperidone (PAL), the 9-hydroxy main metabolite of RIS, is the main causative factor of HPRL. In contrast, aripiprazole (ARI) is a PRL–sparing drug, labeled as the first D2/D3 receptor partial agonist with a unique pharmacological profile. Others have suggested that the 'atypicality' of ARI is most likely combined with its actions on non-DA receptors. Yet, our understanding of the pathophysiology of antipsychotic-induced HPRL is incomplete. This includes the lack of mechanistic (basic) studies, which could help to distinguish the direct effects of PAL and ARI on human lactotrophs from those that might involve altered hypothalamic secretion of DA and other PRL–inhibiting and/or PRL–stimulating factors, as well as the contribution of receptors other than DA to the action of these drugs.
To address these questions, we studied the direct effects of PAL and ARI on lactotroph function, using a primary culture of female rat anterior pituitary cells as a model system. We used both static cultures and perifusion experiments to study the effects of the compounds on cAMP production and PRL secretion. In cells in static cultures, cAMP was measured intracellularly and extracellularly to account for a substantial cAMP release by cAMP transporters expressed in pituitary cells. DA inhibited spontaneous cAMP/calcium signaling and prolactin release. In the presence of DA, PAL rescued cAMP/calcium signaling and prolactin release in a concentration-dependent manner, whereas ARI was only partially effective. In the absence of DA, PAL stimulated cAMP/calcium signaling and prolactin release, whereas ARI inhibited signaling and secretion more potently but less effectively than DA. Forskolin-stimulated cAMP production was facilitated by PAL and inhibited by ARI, although the latter was not as effective as DA. None of the compounds affected prolactin transcript activity, intracellular prolactin accumulation, or growth hormone secretion. The data indicate that PAL has dual hyperprolactinemic actions in lactotrophs by (1) preserving the coupling of spontaneous electrical activity and prolactin secretion in the presence of DA and (2) inhibiting intrinsic D2 receptor activity in the absence of DA, leading to enhanced calcium signaling and secretion. In contrast, ARI acts on prolactin secretion by attenuating, but not abolishing, calcium-secretion coupling (Reference 4).
Use-dependent desensitization of pituitary purinergic P2X2 receptor channels
The purinergic P2X2 receptor (P2X2R) is an ATP–gated non-selective cation channel expressed in various tissues, including pituitary gonadotrophs, where it has important roles in the control of spontaneous and GnRH–stimulated electrical activity, calcium signaling, and gonadotropin secretion. In recent work, our group showed that P2X2R can dilate and desensitize simultaneously and that the desensitization process includes calcium-dependent and -independent mechanisms. During repetitive agonist application, with washout periods of 4–5 minutes, there is a progressive increase in the rates of calcium-dependent desensitization, a phenomenon termed use-dependent desensitization (UDD). Recently, we characterized the desensitization properties of recombinant P2X2R expressed in HEK293 cells. Using, rat, mouse, and human receptors, we showed that two processes contribute to receptor desensitization: bath calcium-independent and -dependent. Calcium-independent desensitization was minor and comparable during repetitive agonist application in cells expressing the full-size receptor, but was pronounced in cells expressing shorter versions of receptors, indicating a role of the C terminus in controlling receptor desensitization. UDD was substantial during initial agonist application and progressively increased during repetitive agonist application in ATP- and bath calcium-concentration–dependent manner. Experiments with substitution of bath sodium with NMDG, a large organic cation, indicated that, in contrast to receptor desensitization, receptor pore dilation was a calcium-independent process. A reduction in the driving force for calcium by changing the holding potential from −60 to +120 mV further indicated that calcium influx through the channel's pore at least partially accounts for UDD. Experiments with various receptor chimeras also indicated that the transmembrane and intracellular domains of P2X2R are required for development of UDD and that decrease in the amplitude of current slows receptor desensitization. Simultaneous calcium and current recording showed development of UDD without a rise in global intracellular calcium concentrations. Combined with experiments with clamping intrapipette concentrations of calcium at various levels, these experiments indicate that domain calcium is sufficient to establish UDD in experiments with whole-cell recordings (Reference 5).
Publications
- Bjelobaba I, Janjic MM, Kucka M, Stojilkovic SS. Cell type-specific sexual dimorphism in rat pituitary gene expression during maturation. Biol Reprod 2015; 93:1-9.
- Tomić M, Bargi-Souza P, Leiva-Salcedo E, Nunes MT, Stojilkovic SS. Calcium signaling properties of a thyrotroph cell line, mouse TaT1 cells. Cell Calcium 2015; 58(6):598-605.
- Bargi-Souza P, Kucka M, Bjelobaba I, Tomić M, Janjic MM, Nunes, MT, Stojilkovic SS. Loss of basal and TRH-stimulated Tshb expression in dispersed pituitary cells. Endocrinology 2015; 156:242-254.
- Kucka M, Tomić M, Bjelobaba I, Stojilkovic SS, Budimirovic DB. Paliperidone and aripiprazole differentially affect the strength of calcium-secretion coupling in female pituitary lactotrophs. Sci Rep 2015; 5:8902.
- Coddou C, Yan Z, Stojilkovic SS. Role of domain calcium in purinergic P2X2 receptor channel desensitization. Am J Physiol Cell Physiol 2015; 308:C729-C736.
Collaborators
- Greti Aguilera, PhD, Program in Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- Dejan B. Budimirovic, MD, PhD, The Johns Hopkins School of Medicine, Baltimore, MD
- Claudio Coddou, PhD, Faculty of Medicine, Universidad Católica del Norte, Coquimbo, Chile
- Zvi Naor, PhD, Tel-Aviv University, Tel-Aviv, Israel
- Hana Zemková, PhD, Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic
Contact
For more information, email stankos@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx or irp.nih.gov/pi/stanko-stojilkovic.


