Neuregulin–ErbB Signaling in Neuronal Development and Psychiatric Disorders

- Andres Buonanno, PhD, Head, Section on Molecular Neurobiology
- Detlef Vullhorst, PhD, Staff Scientist
- Irina Karavanova, PhD, Research Assistant
- Tanveer Ahmed, PhD, Postdoctoral Fellow
- Lalitha Kurada, PhD, Postdoctoral Fellow
- Miguel Skirzewski, PhD, Postdoctoral Fellow
- Larissa Erben, MS, Graduate Student
- Katrina Furth, BS, Special Volunteer
Failure of cortical microcircuits to properly regulate excitatory\inhibitory (E\I) balance is a key feature in the etiology of several developmental psychiatric disorders and neurological diseases, such as schizophrenia, autism, ADHD, and epilepsy. E\I balance is important for the synchronization of the firing pattern of local neuron ensembles, and its dysregulation can degrade cognitive functions and, in extreme cases, result in epileptiform activity. Network activity, in particular oscillatory activity in the gamma-frequency range (30–80 Hz), is altered in psychiatric disorders and may account for their cognitive and behavioral symptoms. We are interested in how Neuregulin (NRG) and its receptor ErbB4, which are both genetically linked to psychiatric disorders, function in an activity-dependent fashion (i.e., experience) in the developing brain to regulate synaptic plasticity, neuronal network activity, and, in rodents, behaviors that model features of psychiatric disorders. We identified a functional interaction between NRG/ErbB4, GABAergic, and dopamine signaling in GABAergic interneurons that is critical for understanding how NRG can regulate E\I balance and synchronous activity in neuronal networks, processes that are important for cognitive functions altered in psychiatric disorders.
Our earlier studies demonstrated that, in the hippocampus and neocortex, expression of ErbB4, the major NRG neuronal receptor, is restricted to GABAergic interneurons—particularly the parvalbumin-positive (Pv+) fast-spiking interneurons that are critical for modulating gamma oscillation induction and strength (i.e., power). Using genetically targeted mouse mutant models, we went on to show that NRG–ErbB4 signaling regulates synaptic plasticity, neuronal network activity, and behaviors associated with psychiatric disorders. More recently, our group has been investigating other aspects of NRG expression throughout the brain, its processing in response to neuronal activity, and its function in distinct neuronal populations of the developing and maturing nervous system. To achieve these goals, we are using a combination of techniques, including electrophysiological recordings in acute brain slices prepared from normal and genetically altered mice, multi-electrode field recordings from brains of freely moving rats, reverse-microdialysis neurochemistry, confocal fluorescence microscopy in fixed and live tissue, proteomics analyses, and behavioral testing. The ultimate goal of this multi-disciplinary approach is to generate holistic models to investigate the developmental impact of genes that modulate E\I balance and neuronal network activity and that consequently affect behaviors and cognitive functions altered in psychiatric disorders.
Unprocessed NRG2 accumulates at endoplasmic reticulum–plasma membrane (ER-PM) junctions, where it is protected from alpha-secretase processing.
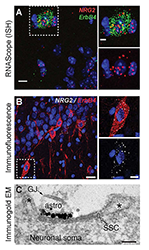
The cellular and molecular processes that promote the conversion of NRG ligands from inactive pro-forms to signaling-competent ligands that can engage ErbB4 receptors to mediate their aforementioned biological effects in the developing and maturing brain remain mostly unknown. To address this major unresolved question, we investigated the role of Neuregulin 2 (NRG2), a Neuregulin isotype that is prominently expressed in the developing postnatal and adult CNS. Using a novel double-labeling in situ hybridization technique (RNAScope) and newly generated monoclonal antibodies, we found that, in the rodent hippocampus, NRG2 mRNA and protein are highly expressed in ErbB4–positive GABAergic interneurons, suggesting that NRG2 can engage in autocrine ErbB4 signaling (Figure 1). Interestingly, we found no evidence of NRG2 protein in axons but instead found that unprocessed proNRG2 accumulates at large somato-dendritic puncta on the plasma membrane. Immunogold electron-microscopy of NRG2 in dissociated hippocampal neurons, performed in collaboration with Susan Cheng, revealed that the puncta are found atop of subsurface cisterns (SSCs)—sites of close endoplasmic reticulum–plasma membrane (ER–PM) apposition (Figure 1). Based on these observations, we hypothesized that pro-NRG2 clustering at SSCs serves to protect the protein from constitutive processing by extracellular sheddases.
Click image to enlarge.
Figure 1. Neuregulin-2 is expressed in ErbB4–positive GABAergic interneurons and accumulates at subsurface cisterns.
A. Double fluorescence ISH of NRG2/Gad67 and NRG2/ErbB4 in the mouse stratum oriens of CA1. B. NRG2 immunoreactivity in ErbB4+ interneurons in rat CA1 strata pyramidale and radiatum. C. Silver-enhanced immunogold EM of a DIV 35 hippocampal neuron with a patch of concentrated label for NRG2 at the plasma membrane atop intracellular membrane stacks characteristic of SSCs.
NMDA receptor function on cortical interneurons promotes proNRG2 processing and, in turn, NRG2 signaling via ErbB4 downregulates NMDAR function on interneurons.
Previous studies reported that clustering of potassium channel Kv2.1 at SSCs is regulated by neuronal activity, in particular via activation of the glutamate receptor NMDAR (N-methyl-D-aspartate receptor). Interestingly, using co-immunofluorescence experiments, we found that proNRG2 puncta reside inside the doughnut-shaped Kv2.1 clusters. Treatment of neuronal cultures with glutamate or NMDA rapidly causes the dispersal of both proteins from the SSC and the processing of proNRG2 by extracellular alpha-secretases to release signaling-competent NRG2.
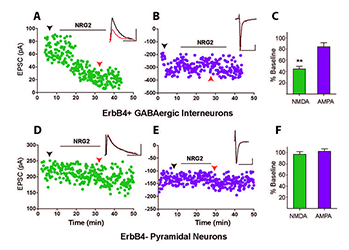
What could be the function of released NRG2 from GABAergic interneurons, which are known to express the ErbB4 receptor? In collaboration with Sanford Markey's group, we used ErbB4 immunoprecipitation from the soluble fraction of metabolically active synaptosomes followed by LC/MS/MS (liquid chromatography coupled with tandem mass spectrometry) to characterize the ErbB4 proteome. Using this approach, we identified the NMDAR GluN2B subunit as an ErbB4–interacting protein following NRG treatment of synaptosomes. The interaction was confirmed in cultured hippocampal neurons, where NRG2 treatment was shown to enhance the internalization of GluN2B–containing, but not GluN2A–containing, NMDARs. Consistent with this observation, we found that NRG2 also caused a dramatic reduction of whole-cell NMDAR currents in dissociated hippocampal ErbB4–positive interneurons, but not in ErbB4–negative glutamatergic neurons. Using whole-cell voltage-clamp recordings in acute medial prefrontal cortical slices, we found that NRG2 selectively reduced NMDAR synaptic currents (EPSCs), and not AMPAR EPSCs, at glutamatergic synapses onto GABAergic interneurons (Figure 2). The results are consistent with the idea that the bidirectlional signaling between NRG2/ErbB4 and NMDAR activity can play a major role in modulating the activity of GABAergic neurons and cortical E/I balance.
Click image to enlarge.
Figure 2. Neuregulin-2 selectively reduces NMDAR currents, but not AMPAR currents, in GABAergic interneurons.
A and B. Representative scatter plots of NMDAR– and AMPAR–mediated evoked EPSCs recorded from an ErbB4–positive GABAergic interneuron and (D and E) recorded from a pyramidal neuron. C and F. Summary graphs of NRG2 effects on NMDA and AMPA eEPSCs. Data normalized to baseline.
Neuregulin-2 ablation results in dopamine dysregulation and severe behavioral phenotypes relevant to psychiatric disorders.
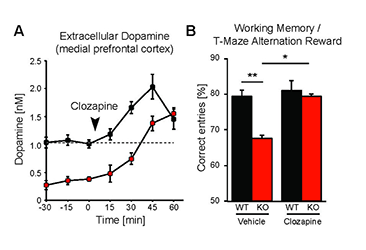
While the neurophysiological and behavioral phenotypes of Nrg1–mutant mice have been investigated extensively, little is known about the function of NRG2 (see above), the closest NRG1 homolog. We found that NRG2 expression in the adult rodent brain does not fully overlap with NRG1 and is more extensive than originally reported, including expression in the striatum and medial prefrontal cortex (mPFC). We therefore generated Nrg2 knockout (KO) mice to study the homolog's function. Nrg2 KO mice have higher extracellular dopamine levels in the dorsal striatum but lower levels in the mPFC, similar to schizophrenia subjects. Nrg2 KOs also performed abnormally in a battery of behavioral tasks relevant to psychiatric disorders. The mutant mice exhibit hyperactivity in the open field, hypersensitivity to amphetamine, deficits in prepulse inhibition, reduced anxiety-like behavior in the elevated plus-maze, and antisocial behavior. Acute administration of clozapine (1mg/kg) increased the concentration of mPFC dopamine and improved performance in the T-maze alteration reward task (Figure 3). We also demonstrated that NMDA receptor synaptic currents in Nrg2 KOs are augmented at hippocampal glutamatergic synapses and more sensitive to ifenprodil, indicating an increased contribution of GluN2B-containing NMDARs. Our findings reveal a novel role for NRG2 in the modulation of behaviors relevant to the core symptoms of schizophrenia (Yan et al., Mol Psychiatry 2015;in review).
Click image to enlarge.
Figure 3. Administration of antipsychotic clozapine restores dopamine levels in pre-frontal cortex of Neuregulin-2 knockout mice and improves performance on a cognitive task.
A. Extracellular dopamine levels in the mPFC of Nrg2 knockout (KO) mice and wild-type (WT) littermates before and after clozapine injection (arrowhead). B. Poorer performance by Nrg2 KO mice in a T-maze reward alternation task than by WT littermates (left) can be restored by clozapine treatment (right).
Neuregulin 3 (NRG3) is a dual-pass transmembrane protein targeted to the axons in an activity-dependent process.
NRGs are encoded by different genes in the brain (NRG1-3), which are transcribed from different promoters and alternatively spliced to generate a large variety of isoforms. In contrast to NRG1, little is known about the cellular expression and functions of NRG2 and NRG3 in the brain. Our study, funded by an NICHD Director's Award, aimed to determine the cellular and subcellular distribution pattern of NRG3 and its function in neurons. Using a novel triple-fluorescence in situ hybridization technique (RNAScope), we found that NRG3 transcripts are widely expressed in the adult mouse brain and relatively more abundant in V-Glut1–positive excitatory cells than in GAD1–positive inhibitory interneurons. Contrary to prior studies reporting NRG3 as a single-pass type I membrane protein, our analysis revealed that NRG3 is a dual-pass transmembrane-domain (TMD) protein. Interestingly, the secondary structure and axonal distribution of NRG3 is extremely similar to the CRD (cysteine-rich domain, type III) variant of NRG1 (CRD-NRG1) but differs significantly from other NRG1 variants (type I and II) and NRG2 that accumulate at somatodendritic compartments (Ahmed et al., manuscript in preparation).
Mesocortical and nigrostriatal DA function in mice with targeted mutations of ErbB4 in parvalbumin-positive (Pv+) and tyrosine hydroxylase–positive (TH+) neurons
Dysfunctional NRG–ErbB4 signaling in the hippocampus, pre-frontal cortex (PFC), and striatum may contribute to alterations in dopamine (DA) function associated with several schizophrenia symptoms. Because we had shown that NRG1 acutely increases extracellular DA levels to regulate LTP and gamma oscillations, and that ErbB4 expression is confined to GABAergic interneurons (cortex) and TH+ mesocortical DA, we are investigating the relative role NRG/ErbB4 signaling in the two different neuronal populations. To this end, we are measuring how NRG signaling affects extracellular DA levels in the PFC, hippocampus, and striatum in mice harboring targeted mutations of the receptor in either Pv+ or TH+ neurons. We also are comparing their behaviors to begin unraveling the role of Neregulin-ErbB4 signaling in Pv+ interneurons vs. TH+ terminals in regulating schizophrenia-associated behaviors (Skirzewski et al., manuscript in preparation). In collaboration with Alon Shamir and Idit Golani, we analyzed the effects of developmentally disrupting the NRG-ErbB signaling pathway on dopamine balance and behaviors. Alteration of ErbB signaling during adolescence were found to affect the dopaminergic system and to coincide in the adult with deficits in learning and hedonic behaviors. The results suggest a possible role of the NRG–ErbB pathway in the development of cognitive skills.
Functional analysis of the extra-synaptic ErbB4 receptor proteome using proteomics approach; identifying an interaction with GABA-A receptors
Most studies on NRG–ErbB4 signaling focused on the receptor that accumulates at glutamatergic post-synaptic densities (PSDs). However, little research has been performed on the pool of extra-synaptic ErbB4 receptors that may constitute up to 80% of the total receptor population in cortical and hippocampal interneurons. In collaboration with Sanford Markey's group, our laboratory used ErbB4 immunoprecipitation from the soluble fraction of metabolically active synaptosomes followed by LC/MS/MS to characterize the ErbB4 proteome. Using this approach, we identified the GABA-A receptor α1 subunit (GABAR α1) as an ErbB4–interacting protein. Consistent with this observation, we found that GABAR α1 receptors are highly expressed in ErbB4–positive fast-spiking Pv+ in the hippocampus and that Erbb4–knockout mice have reduced GABAR α1 levels. Remarkably, we found that this novel ErbB4 signaling pathway, which suppresses postsynaptic GABAR currents on GABAergic interneurons, acts independently of ErbB4s canonical receptor tyrosine kinase (RTK) activity. While the effects of NRG on GABAR α1 internalization do not require RTK activity, the ErbB4 protein is necessary for clathrin-mediated endocytosis and reduction of GABA receptor IPSCs (inhibitory postsynaptic currents).
Dopamine regulation of prefrontal cortical gamma oscillations in freely moving rats
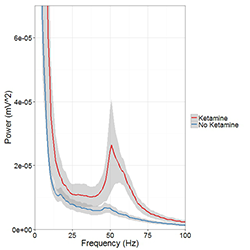
Mounting evidence suggests that gamma oscillations are atypically high at baseline in disorders that affect attention such as schizophrenia and ADHD. A lower evoked gamma oscillatory power in these subjects than in healthy controls may constitute an endophenotype associated with risk for developing the psychiatric disorders. Based on these observations, we hypothesized that drugs targeting either the D4 or ErbB4 receptors may have therapeutic potential in treating cognitive deficits in patients with schizophrenia. To examine abnormal oscillatory patterns and connect them with the firing patterns of single neurons, we, in collaboration with Judith Walters, are using multi-electrode recordings from the medial prefrontal cortex and dorsomedial thalamus of rats acutely treated with ketamine to analyze the effects of D4– and ErbB4–targeting drugs on gamma oscillations in this mouse model with "face validity" for schizophrenia (Figure 4).
Click image to enlarge.
Figure 4. Ketamine raises gamma (40-65Hz) frequency power in the medial prefrontal cortex (mPFC) during controlled movement.
Multi-electrode recordings in the mPFC revealed an over three-fold increase in gamma (40–65Hz) frequency power while rats walked counter-clockwise at 9–10 revolutions per minute on a circular treadmill. Blue traces come from an epoch 30 minutes before ketamine administration, and the red trace comes from epochs 15 minutes after ketamine administration.
Additional Funding
- NICHD DIR Director's Investigator Awards (RRC# D-14-07)
- Bench-to-Bedside Award
Publications
- Vullhorst D, Mitchell RM, Keating C, Roychowdhury S, Karavanova I, Tao-Cheng JH, Buonanno A. A negative feedback loop controls NMDA receptor function in cortical interneurons via neuregulin 2/ErbB4 signalling. Nat Commun 2015;6:7222.
- Golani I, Tadmor H, Buonanno A, Kremer I, Shamir A. Disruption of the ErbB signaling in adolescence increases striatal dopamine levels and affects learning and hedonic-like behavior in the adult mouse. Eur Neuropsychopharmacol 2014;24:1808-1018.
- Furth KE, Mastwal S, Wang KH, Buonanno A, Vullhorst D. Dopamine, cognitive function, and gamma oscillations: role of D4 receptors. Front Cell Neurosci 2013;7:102 (1-19).
- Penzes P, Buonanno A, Passafaro M, Sala C, Sweet RA. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J Neurochem 2013;126:165-182.
- Mitchell RM, Janssen MJ, Karavanova I, Vullhorst D, Furth K, Makuskey A, Markey S, Buonanno A. ErbB4 receptor reduces synaptic GABAA currents independently of its tyrosine kinase activity. Proc Natl Acad Sci USA 2013;110:19603-19608.
Collaborators
- Jung Hwa (Susan) Cheng, PhD, EM Facility, NINDS, Bethesda, MD
- Catherine Fenster, PhD, The College of Wooster, Wooster, OH
- Idit Golani, MD, Technion–Israel Institute of Technology, Haifa, Israel
- Luis Hernández, MD, Universidad de los Andes, Mérida, Venezuela
- Ilana Kremer, MD, The Ruth and Bruce Rappaport Faculty of Medicine, Technion–Israel Institute of Technology, Haifa, Israel
- John Lisman, PhD, Brandeis University, Waltham, MA
- Sanford P. Markey, PhD, Laboratory of Neurotoxicology, NIMH, Bethesda, MD
- Jörg Neddens, PhD, JSW Life Sciences, Grambach, Austria
- Alon Shamir, PhD, Mazra Mental Health Center, Akko, Israel
- Judith R. Walters, PhD, Experimental Therapeutics Branch, NINDS, Bethesda, MD
Contact
For more information, email buonanno@mail.nih.gov or visit http://smn.nichd.nih.gov.


