Receptors and Actions of Peptide Hormones and Regulatory Proteins in Endocrine Mechanisms

- Maria L. Dufau, MD, PhD, Head, Section on Molecular Endocrinology
- Raghuveer Kavarthapu, PhD, Postdoctoral Fellow
- Peng Zhao, PhD, Postdoctoral Fellow
We investigate the molecular basis of peptide hormone control of gonadal function, with particular emphasis on the structure and regulation of the genes encoding the luteinizing hormone receptor (LHR) and prolactin (PRL) receptor (PRLR). We also investigate the regulatory mechanism(s) involved in the progress of spermatogenesis and the control of Leydig cell (LC) function. Our studies focus on the regulation of human LHR transcription (nuclear orphan receptors, epigenetics, DNA methylation, second messengers, repressors, corepressors, and coactivators), as well as on the multiple-promoter control of hPRLR gene transcription. We are elucidating the functions of two inhibitory short forms of prolactin receptors and their impact on the long form of the receptor as well as their relevance to physiological regulation and breast cancer. We also investigate novel gonadotropin-regulated genes relevant to the progression of testicular gametogenesis, LC function, and other endocrine processes. We focus on the function and regulation of the gonadotropin-regulated testicular RNA helicase (GRTH/DDX25), an essential post-transcriptional regulator of spermatogenesis that was discovered, cloned, and characterized in our laboratory. The various functions of GRTH/DDX25 provide a fertile ground for the development of a male contraceptive.
The luteinizing hormone receptor
The luteinizing hormone receptor (LHR) is expressed primarily in the gonads, where it mediates luteinizing hormone (LH) signals that regulate cyclic ovarian changes or testicular function. The LRH transcription gene is regulated by complex and diverse networks, in which coordination and interactions between regulatory effectors are essential for silencing/activation of LHR expression. The proximal Sp1 site of the promoter recruits histone (H) deacetylases and the Sin3A corepressor complex that contributes to the silencing of LHR transcription. Site-specific acetylation/methylation-induced phosphatase release serves as an on switch for Sp1 phosphorylation at Ser641, which causes p107 repressor release from Sp1, recruitment of Transcription Factor II B (TFIIB) and RNA polymerase II (Pol II), and transcriptional activation. Maximal derepression of the gene is dependent on DNA demethylation of the promoter, H3/acetylation, and HDAC/Sin3 A release. Positive Cofactor 4 (PC4) has an important role in the formation assembly of the preinitiation complex (PIC) in trichostatin A (TSA)–mediated LHR transcription (Kavarthapu et al., Endocrinology 2013;154:2200). It is recruited by Sp1 following TSA treatment and acts as a coactivator. However, PC4 does not participate in TSA release of phosphatases, Sp1 phosphorylation, or release of repressor or complexes. Although TFIIB recruitment is dependent on PC4, we ruled out TFIIB as its direct target and acetylation of PC4 in the activation process. However, we demonstrated TSA–induced acetylation of PC4–interacting proteins, identified as acetylated H3 by mass spectrometry, and PC4's presence in the complex in association with chromatin at the promoter was demonstrated by ChIP/reChIP. The role of these interactions on chromatin structure and their participation in the assembly of the PIC and transcriptional activation are under investigation. Immunoprecipitated flag-tagged PC4/H3 complexes in transfected MCF-7 cells analyzed by immunoblotting revealed H3-acetylated at K9, 14, 18, 23, 27 and 36 pull-down by the Flag Ab. To elucidate the physiological impact of PC4 on Sp1–directed transcription in gonads, we are generating a PC4-floxed mice to be bred with transgenic mice expressing tissue-specific Cyp17 Cre (Cyp17 is a steroidogenic enzyme specifically expressed in Leydig cells and ovarian cells).
Gonadotropin-regulated testicular RNA helicase
Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) is a testis-specific member of the DEAD-box family of RNA helicases present in LCs and meiotic germ cells. It is a multi-functional protein essential for the completion of spermatogenesis. Males lacking GRTH are sterile owing to the absence of sperm as a result of the failure of round spermatids to elongate. Besides its intrinsic RNA helicase activity, it is a shuttling protein that exports specific mRNAs from the nucleus to cytoplasmic sites. Our studies demonstrated the essential participation of GRTH–mediated export/transport of mRNAs in the structural integrity of the Chromatoid Body (storage/processing of mRNAs) and their transit/association to actively translating polyribosomes, where GRTH may regulate translational initiation of genes. We identified mRNAs that are associated with GRTH at polysomal sites of spermatocytes and round spermatids of the mouse testis. The reduction in mRNAs associated at these sites in studies comparing knockout (KO) with wild-type (WT), which is not detected at the total cellular level but evident in the cytoplasm with abolition of protein expression, reflects the importance of the transport function of GRTH to relevant sites and underscores its impact on protein synthesis. The multiple functions of GRTH to regulate post-transcriptional events, including processing, exporting, and storage of RNA, have been viewed as essential for controlling the availability of specific transcripts such as Tp1/2 (transition proteins 1/2) and Prm1/2 (protamines 1/2) for translation during the progression of spermatogenesis. These chromatin remodelers, which are essential for spermatid elongation and completion of spermatogenesis and whose RNAs associate with GRTH, failed to express in GRTH–null mice with impaired nuclear export of mRNA. Our recent studies provided insights into the association of TP2 expression via binding of its mRNA to GRTH protein. Two of the conserved RNA binding motifs of the DEAD-box family of RNA helicases Ia (PTRELA) and V (ARGID) are essential for GRTH binding to 3′ UTR of Tp2 mRNA. Nucleotide sequences within 1–47 and 78–127 downstream of the TGA stop codon of the Tp2 transcript are important for binding to GRTH (Reference 3). The study provides the basis for a deeper understanding of the contribution of GRTH to the regulation of the expression of genes as a component of mRNP particles.
GRTH is regulated by LH through androgen at the transcriptional level in LCs (direct) and germ cells (indirect), where GRTH's expression is both cell- and stage-specific. The helicase displays a novel negative autocrine control of androgen production in LCs by preventing overstimulation of the LH–induced androgen pathway through enhanced degradation of the StAR protein (Steroidogenic Acute Regulatory Protein) and, in this manner, controls the degree of cholesterol transport to the mitochondria and its availability for steroidogenesis. Our studies revealed the mechanism by which androgen (A)/androgen receptor (AR) regulates the expression of the GRTH gene in the LC. Through its activation of GRTH transcription, androgen/AR signaling in LCs participates in an autocrine regulatory mechanism with a major impact on LC steroidogenic function.
Our development of transgenic mice model carrying a GRTH 5′ flanking region–GFP reporter provided a unique in vivo system for differential elucidation of regulatory regions upstream in the GRTH gene that direct its expression (upstream) in germ cells (pachytene spermatocytes and round spermatids) and downstream in LCs and its regulation by A/AR (directly) in LC and indirectly in germ cells (Kavarthapu at al., Endocrinology 2013;154:2200). We identified a functional binding site for the germ cell–specific transcription factor Germ Cell Nuclear Factor (GCNF) in the GRTH 5′ UTR distal region (Reference 4). GCNF is a member of the orphan nuclear receptor superfamily that is expressed in nucleus of spermatocytes and spermatids of adult mice. By contrast, the proximal region of GRTH 5′ UTR directs basal GRTH expression and androgen-induced intracrine expression in LCs through a functional androgen-response element (ARE). In the transgenic animal model, the AR antagonist Flutamide blocked GRTH–GFP expression in LCs and germ cells (GC), demonstrating direct (intracrine regulation by androgen/AR in LCs) and indirect effects of androgen/AR in GC, which do not express AR, through paracrine regulation by androgen/AR in Sertoli cells (Reference 4). Upon treatment with Flutamide, GCNF protein expression was significantly reduced in GC, indicating the presence of a regulatory network for an androgen GCNF–upstream 5′ UTR sequence to regulate GRTH expression in GC. ChIP analysis further demonstrated the association of GCNF with the GRTH sequence spanning the GCNF–binding site; the interaction was abolished in round spermatids by antagonist treatment. Flutamide treatment of WT mice caused selective reduction of GCNF and GRTH in round spermatids. GCNF knock-down in seminiferous tubules (dark zone, round spermatid–rich) caused a reduction in GRTH expression. Exposure of tubules to Flutamide caused a reduction in GCNF and GRTH expression while androgen exposure induced a significant increase. Moreover, GRTH associates with GCNF mRNA. Its absence in GRTH–null mice and in seminiferous tubules with GRTH knock-down caused increase on GCNF expression and mRNA stability (12 h KO versus 5 h WT), indicative of negative autocrine post-transcriptional regulation of GCNF by GRTH (Figure 1). The studies clearly established that GCNF present in germ cells is regulated by androgen in round spermatids and that this transfactor in turn regulates GRTH at the transcriptional level. Such regulation, most likely from Sertoli cells, could result from classical or non-classical A/AR–mediated actions. These in vivo and in vitro models link androgen action to GC through GCNF, as a transcriptionally and post-transcriptionally regulated transfactor that controls transcription/expression of GRTH. The studies provided, for the first time, a connection between androgen action and two relevant germ cell genes, GCNF and GRTH, which are essential for the progress of spermatogenesis, and established their relationship (Figure 1). Studies on the molecular regulatory aspects of androgen on GCNF transcription/expression and function provide valuable links and clearly facilitate what could be a quite difficult search for identification of A/AR–mediated regulated gene product(s) in Sertoli cells affecting germinal cell function and spermatogenesis.
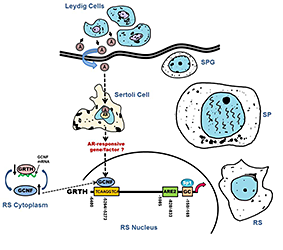
Click image to enlarge.
Figure 1. Germ Cell Nuclear Factor (GCNF) regulates transcription of gonadotropin-regulated testicular helicase (GRTH/DDX25) in testicular germ cells—the androgen connection.
GCNF regulation of GRTH transcription/expression in testicular germ cells links androgen action and germ cell activation. Androgen (A) synthesized and released from Leydig cells (interstitial testis compartment) binds to androgen receptors (AR) in Sertoli cells (somatic cells located in tubule compartment; they provide structural support and nutrients to germ cells). A/AR complex enters into the nucleus of Sertoli cells, where it activates AR–responsive genes/factors (classical pathway). In addition, the non-classical pathway could mediate this GRTH activation by the transfactor GCNF. Both pathways could lead to activation of kinases and/or phosphatases or other modifiers for recruitment of coactivators or release of repressors of GCNF to induce transcription/expression of GRTH. Sertoli-mediated events/signals in response to androgen are in turn passed onto germinal cells [Round spermatids (RS)], where they activate GCNF, a germinal cell–specific transfactor, that binds to the upstream 5′ region of the GRTH gene and promotes its transcription/expression. Association of GRTH with GCNF mRNA at cytoplasmic sites and its inhibitory effect on GCNF message stability indicate that the helicase plays a role in the regulation of is own transcriptional regulator in germ cells (Reference 4). SPG, spermatogonia; SP, spermatocytes.
The prolactin receptor
The human prolactin receptor (PRLR) mediates the diverse cellular actions of prolactin (PRL). PRL plays a major role in the proliferation and differentiation of breast epithelium and is essential for stimulation and maintenance of lactation. It has also been implicated in the development of breast cancer, tumor growth, and chemo-resistance. PRLR expression is controlled at the transcriptional level by several promoters (one generic, [PIII], and five human-specific [hPN1–hPN5]), which were defined and characterized in our laboratory. Each promoter directs transcription/expression of a specific non-coding exon 1 (E1–3, hEN1–hEN5), a common non-coding exon 2, and coding exons (E3–E11). Transcription of PRLR in breast cancer cells is directed by the preferentially utilized PIII, which lacks an estrogen-response element. BRET (bioluminescence resonance energy transfer) studies revealed ERα constitutive homodimers. Complex formation of the ERα dimer (non-DNA bound) with the transcription factors Sp1 and C/EBPβ, bound to their cognate sites at the PIII promoter, is required for basal (constitutive ERα homodimers) and E2–induced transcriptional activation/expression of the human PRLR gene (Reference 1).
In tumoral breast PRL causes cell proliferation by activating its cognate receptor. Exacerbation of PRL's actions in breast cancer resulting from increase receptor expression can explain resistance to estrogen inhibitors in breast cancer. Our studies in MCF7 and T47D cells reveal stimulation by PRL of PRLR transcription, mRNA, and protein in the absence of E2, which is abolished by mutation of a GAS site (Stat5 DNA-recognition motif), by Stat5 siRNA, or by an ER antagonist (ICI). This indicates the participation of the ERα in PRLR transcription via PRL/PRLR/Stat5. PRL/PRLR induces phosphorylation of ERα through the JAK2/PI3K/MAPK/ERK– and the HER2–activated pathways (Reference 1). Increased recruitment of phospho-ERα to Sp1 and C/EBPβ bound at promoter sites is essential for PRL–induced receptor transcription. Direct evidence for local actions of PRL independent of E2 is provided in the up-regulation of PRLR transcription/expression via the Stat5/ER activation loop with requisite participation of signaling mechanisms (Reference 2). These studies, which demonstrated a central role ERa in PRLR receptor up-regulation, are of relevance in states refractory to aromatase inhibitors, in which cancer progression can be fueled by endogenous PRL. Therapies that inhibit the function of PRL or PRLR, combined with inhibitors of various signaling pathways, could reverse resistance in breast cancer. Moreover, a combination therapy targeting ER and PRLR directly can offer an additional avenue to eliminate constitutive activation of ER and of PRLR by endogenous prolactin.
In current studies we are addressing the role of EGFR in the up-regulation of the PRLR, given that most breast cancers that become resistant to endocrine therapy have elevated expression/activation of EGFR and its family member ERBB2. Other studies demonstrated the essential role of the D1 domain of the PRLR short-form structure and its inhibitory action on PRL–induced long-form function. Changes in PRLR structure and dimerization affinity are triggered by single mutations in D1, providing avenues for breast cancer treatment (Reference 2). Collaborative studies demonstrated that indirect cross-talk of membrane initiates GnRH/GnRHR and E2/ERa signaling in immortalized GnRH neurons (GT1–7cells). Although there was no evidence of direct interaction between ERs and GnRH-R, GnRH agonist reduced ERa homodimerization, which led us to postulate that signaling events initiated by GnRH agonist influence ER dimerization and/or availability (Reference 5).
Publications
- Kavarthapu R, Tsai Morris CH, Dufau ML. Prolactin induces up-regulation of its cognate receptor in breast cancer cells via transcriptional activation of its generic promoter by cross-talk between ERa and STAT5. Oncotarget 2014; 5:9079-9091.
- Kang JH, Hassan SA, Zhao P, Tsai Morris CH, Dufau ML. Impact of subdomain D1 of the short form S1b of the human prolactin receptor on its inhibitory action on the function of the long form of the receptor induced by prolactin. Biochim Biophys Acta 2014; 1840:2272-2280.
- Yang, R, Tsai-Morris CH, Kang JH, Dufau ML. Elucidation of RNA binding regions of gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) to transcripts of a chromatin remodeling protein essential for spermatogenesis. Horm Mol Biol Clin Investig 2015; 22:119-130.
- Kavarthapu R, Dufau ML. Germ Cell Nuclear (GCNF/RTR) regulates transcription of gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) in testicular germ cells—the androgen connection. Mol Endocrinol 2015; 29(12):1792-804.
- Chason RJ, Kang J-H, Gerkowicz SA, Dufau ML, Catt KJ, Segars JH. GnRH agonist reduces estrogen receptor dimerization in GT1-7 cells: Evidence for cross-talk between membrane-initiated estrogen and GnRH signaling. Mol Cell Endocrinol 2015; 404:67-74.
Collaborators
- Sergio A. Hassan, PhD, Center for Molecular Modeling, Division of Computational Bioscience, NIH, Bethesda, MD
- James M. Pickel, PhD, Transgenic Core Facility, NIMH, Bethesda, MD
- James H. Segars, MD, Program in Reproductive and Adult Endocrinology, NICHD, Bethesda, MD
Contact
For more information, email dufau@helix.nih.gov or visit irp.nih.gov/pi/maria-dufau.


