Thyroid Hormone Regulation of Vertebrate Postembryonic Development

- Yun-Bo Shi, PhD, Head, Section on Molecular Morphogenesis
- Liezhen Fu, PhD, Staff Scientist
- Thomas Miller, PhD, Postdoctoral Intramural Research Training Award Fellow
- Morihiro Okada, PhD, Visiting Fellow
- Julia Rodiger, PhD, Visiting Fellow
- Yuki Shibata, PhD, Visiting Fellow
- Luan Wen, PhD, Visiting Fellow
- Nga Luu, MS, Biologist
- Dan Su, MD, Adjunct Scientist
This laboratory investigates the molecular mechanisms of thyroid hormone (TH) function during postembryonic development. The main model is the metamorphosis of Xenopus laevis and X. tropicalis, two highly related species, that offer unique but complementary advantages. The control of their developmental process by TH offers a paradigm to study gene function in postembryonic organ development. During metamorphosis, diverse organs undergo vastly different changes. Some, like the tail, undergo complete resorption, while others, such as the limb, are developed de novo. The majorities of larval organs persist through metamorphosis but are dramatically remodeled to function in a frog. For example, tadpole intestine is a simple tubular structure consisting primarily of a single layer of larval epithelial cells. During metamorphosis, it is transformed into an organ with a multiply folded adult epithelium surrounded by elaborate connective tissue and muscles, a process that occurs through specific larval epithelial cell death and de novo development of the adult epithelial stem cells followed by their proliferation and differentiation. The wealth of knowledge from past research and the ability to manipulate amphibian metamorphosis both in vivo, by using genetic approaches or hormone treatment of whole animals, and in vitro in organ cultures offer an excellent opportunity to first study the developmental function of TH receptors (TRs) and the underlying mechanisms in vivo and second to identify and functionally characterize genes that are critical for organogenesis, in particular those responsible for the formation of adult organ-specific stem cells during postembryonic development in vertebrates. Our studies revealed likely conserved mechanisms in adult intestinal stem cell development in vertebrates, which prompted us to develop a mouse model to complement the amphibian system for gene knockout studies.
Adapting TALEN and CRISPR technologies for studying GENE function during metamorphosis
In the past, gene knockout and knockdown in Xenopus tadpoles had been very difficult, if not impossible. We recently successfully adapted the TALEN (transcription activator-like effector nucleases, artificial restriction enzymes) and CRISPR (clustered regularly interspaced short palindromic repeats, a gene-editing tool) technologies to knockdown endogenous genes in Xenopus tadpoles by microinjecting TALEN and CRISPR RNAs into fertilized eggs (References 1 and 2). Importantly, we showed that we could achieve very high efficiencies of mutations in the target genes in the resulting premetamorphic tadpoles, making it possible to carry out functional studies directly on these tadpoles, the so called F0 genetic studies, which eliminates the need for F1 or F2 generation animals, thus allowing much faster determination of gene function. This has enabled us to investigate the roles of TRα and a histone methyltransferase, a likely TR coactivator as described below.
A novel function for TRα during development
We developed a TALEN that could mutate the X. tropicalis gene encoding TRα with over 90% efficiency by injecting TALEN mRNAs into fertilized eggs, making it possible to analyze the role of TRα in the resulting F0 animals (Reference 3). Consistent with our dual function model for TR, we observed that knocking down TRα accelerated animal development, with the knockdown animals reaching the onset of metamorphosis earlier (Figure 1). On the other hand, they were resistant to exogenous triiodothyronine (T3) treatment and had delayed natural metamorphosis. Thus, our studies directly demonstrated a critical role of endogenous TRα both in mediating the metamorphic effect of T3 during metamorphosis and in preventing precocious initiation of metamorphosis when T3 is absent. Surprisingly, we also found that TRα knockdown enhanced tadpole growth in premetamorphic tadpoles. This novel function of unliganded TRα (as little T3 is present during premetamorphosis) appears to be attributable to increased growth hormone gene expression. Further analyses suggest that the two functions of unliganded TRα during metamorphosis, i.e., regulating tadpole growth rate and the time to reach the onset of metamorphosis, are independent of each other. While this is the first direct evidence for a critical role of unliganded TR in vertebrate development, TRa knockout studies suggest that unliganded TRa is important for postembryonic regulation of heart rate and gene expression. Similarly, deleting the gene encoding the T3-inactivating enzyme type 3 deiodinase causes auditory defects, arguing for the importance of maintaining a very low level of T3, which would lead to more unliganded TR, for cochlear development. Thus, unliganded TR may also be important for postembryonic development in mammals.
Click image to enlarge.
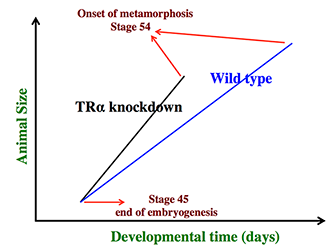
Figure 1. Schematics showing the dual effects of TRα knockdown on premetamorphic development in Xenopus tropicalis
TRα knockdown has little effect on embryogenesis, and the resulting tadpoles are normal by feeding stage (stage 45). Once feeding begins, the animals grow differently, with the knockdown ones growing faster, and thus larger when compared to wild-type siblings at the same age (in days) (compare the vertical axis values of the lines for the knockdown and wild-type animals at any given position along the horizontal axis between stages 45 and 54). The knockdown animals also have faster development, reaching developmentally more advanced stages than do wild-type siblings at the same age (in days). Thus, the knockdown animals reach stage 54, the onset of metamorphosis, at younger age (see the horizontal axis locations for the upper end of the lines). Interestingly, when the animals at stage 54 are compared, the wild-type ones are larger than the knockdown siblings, even though the latter grow faster. This is because the wild-type animals take longer to reach metamorphosis (stage 54). The extra growth time needed to reach stage 54 enables the wild-type ones to catch up and surpass the knockdown ones in size. The results indicate that the effects of TR on growth and development are not dependent on each other (as otherwise, the size of the animals at stage 54 would be identical between wild type and knockdown).
Identification of the histone methyltransferase Dot1L as a direct target gene of TR and its essential role in premetamorphic tadpole growth
We earlier identified Dot1L, the only histone methyltransferase (HMT) capable of methylating histone H3K79 in vitro, as a direct target gene of T3. Interestingly, the level of H3 lysine79 (H3K79) methylation is strongly enhanced by T3 at TR target genes, suggesting that Dot1L is upregulated by TR and, in turn, functions as a TR coactivator. To investigate Dot1L function, we generated a Dot1L–specific TALEN that was extremely efficient in mutating Dot1L when expressed in X. tropicalis fertilized eggs, creating animals with almost no Dot1L and little H3K79 methylation (Reference 1). We observed that Dot1L knockdown had no apparent effect on embryogenesis, whereas it severely retarded tadpole growth and led to tadpole lethality before metamorphosis. The findings suggest that Dot1L and H3K79 methylation play an important role in tadpole growth and development prior to metamorphosis. Our analyses further revealed interesting similarities between Xenopus and mouse development and suggested the existence of two separate phases of vertebrate development with distinct requirements for epigenetic modifications.
Requirement of T3-induced Sonic hedgehog paracrine signaling for adult intestinal development
We have demonstrated that T3 activates the transcription of the Sonic hedgehog (shh) gene directly at the transcriptional level during intestinal metamorphosis and that exogenous Shh promotes cell proliferation in intestinal organ cultures. To determine the role of Shh during metamorphosis, we treated premetamorphic tadpoles with the Shh inhibitor cyclopamine and observed that it specifically inhibited the expression of the Shh response genes snai2 and twist1 (Reference 4). More importantly, cyclopamine reduced the proliferation of both developing adult stem cells in the epithelium and cells in the other intestinal tissues at the climax of metamorphosis, leading to delayed/incomplete remodeling of the intestine at the end of metamorphosis. We further revealed that both Snai2 and Twist1 were strongly upregulated specifically in the connective tissue during intestinal metamorphosis, suggesting that Shh signals the connective tissue to promote stem cell proliferation and the formation of the adult intestine. Consistently, we found that the Shh receptor Patched (Ptc)-1 and the signaling protein Smoothened (Smo), as well as the downstream transcription factors Gli1, Gli2, and Gli3, were all transiently up-regulated in the mesenchymal tissues, but not the epithelium, where Shh was induced by T3, during intestinal metamorphosis. We also showed, in intestinal organ cultures, that overexpression of Shh enhanced the expression of Ptc-1, Smo, and Glis even in the absence of T3, indicating that Shh regulates the components of its own pathway during intestinal remodeling. Thus, Shh signaling induced by T3 is required for adult intestinal stem-cell proliferation and functions via a paracrine mechanism.
Essential role of System L1 amino acid/thyroid hormone transporter in mouse development
The frog model offers a unique opportunity to identify and functionally characterize novel genes that are involved in the development of the adult intestine, particularly the adult stem cells. Interestingly, in all vertebrates, the formation/maturation of the adult intestine takes place around the time when plasma T3 levels are high. T3 or TR deficiency in mouse leads to abnormal intestinal morphology and a reduction in stem cell proliferation in the adult, and liganded TRa1 regulates stem cells during mouse intestinal maturation and homeostasis. Furthermore, we showed that many genes with peak levels of expression at the climax of metamorphosis are upregulated during mouse intestinal maturation. Our earlier studies suggest a conserved role of the histone 4 methyltransferase PRMT1 in stem cell development across vertebrates. Thus, we hypothesized that the formation of adult intestinal stem cells utilize conserved mechanisms, including regulation by T3 and the involvement of conserved T3 target genes during vertebrate development. To test our hypothesis, we initiated studies to take advantage of the ability to generate conditional knockout mice to investigate the developmental roles of the mouse homologs of the novel stem cell genes that we have discovered.
One of the genes, Lat1, that we previous discovered as a T3 response gene in the intestine during metamorphosis encodes the catalytic subunit (light chain) of the heterodimeric System L1 amino acid transporter. Interestingly, when LAT1 was co-expressed with the heavy chain (CD98hc) of the transporter, it mediated the intracellular uptake of T3 and, more importantly, enhanced transcriptional activation by TR in the presence of T3. Thus, LAT1 is activated by T3 and, in turn, may function to enhance the effect of T3 on adult intestinal development. We since showed that targeted disruption of the gene encoding CD98hc to specifically inactivate the heavy chain's ability to form a functional transporter with LAT1 led to mouse embryonic lethality, suggesting that LAT1 transporter activity is critical for organogenesis in mouse. In addition, we generated a mouse line with Floxed Lat1 gene, which, when crossed with a mouse expressing the Cre recombinase, leads to the inactivation of Lat1 gene. When it was crossed with mice expressing Cre driven by a global promoter, we observed that homozygous Lat1 knockout animals were embryonic lethal (Reference 5), consistent with the findings on Cd98hc mutant animals. The findings suggest that LAT1 is critical for mouse organogenesis. Thus, it will be of interest to investigate whether LAT1 affects the development of the adult mouse intestinal stem cells by functioning as a T3 transporter.
Additional Funding
- Morihiro Okada is supported by a JSPS fellowship.
Publications
- Wen L, Fu L, Guo X, Chen Y, Shi Y-B. Histone methyltransferase Dot1L plays a role in postembryonic development in Xenopus tropicalis. FASEB J 2015 29:385-393.
- Wang F, Shi Z, Cui Y, Guo X, Shi Y-B, Chen Y. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci 2015 5:15.
- Wen L, Shi Y-B. Unliganded thyroid hormone receptor a controls developmental timing in Xenopus tropicalis. Endocrinology 2015 156:721–734.
- Wen L, Hasebe T, Miller TC, Ishizuya-Oka A, Shi Y-B. A requirement for hedgehog signaling in thyroid hormone-induced postembryonic intestinal remodeling. Cell Biosci 2015 5:13.
- Poncet N, Mitchell FE, Ibrahim AFM, McGuire VA, English G, Arthur SC, Shi Y-B, Taylor PM. The catalytic subunit of the System L1 amino acid transporter (Slc7a5) facilitates nutrient signaling in mouse skeletal muscle. PLoS One 2014 9:e89547.
Collaborators
- Doreen A. Cantrell, PhD, University of Dundee, Dundee, UK
- Yonglong Chen, PhD, Guangzhou Institute of Biomedicine and Health, Guangzhou, China
- Atsuko Ishizuya-Oka, PhD, Nippon Medical School, Tokyo, Japan
- Peter Taylor, PhD, University of Dundee, Dundee, UK
Contact
For more information, email shi@helix.nih.gov or visit http://smm.nichd.nih.gov.


