Protein Sorting in the Endosomal-Lysosomal System

- Juan S. Bonifacino, PhD, Head, Section on Intracellular Protein Trafficking
- Rafael Mattera, PhD, Staff Scientist
- Xiaolin Zhu, RN, Technician
- Raffaella De Pace, PhD, Visiting Fellow
- Atena Farkhondeh, PhD, Visiting Fellow
- David Gershlick, PhD, Visiting Fellow
- Carlos M. Guardia, PhD, Visiting Fellow
- Morie Ishida, PhD, Visiting Fellow
- Rui Jia, PhD, Visiting Fellow
- Tal Keren-Kaplan, PhD, Visiting Fellow
- Sang-Yoon Park, PhD, Visiting Fellow
- Jing Pu, PhD, Visiting Fellow
- Amra Saric, PhD, Visiting Fellow
- David Kolin, BS, Postbaccalaureate Student
- Jeffrey Mercurio, BS, Postbaccalaureate Student
- Abby Moskowitz, BS, Postbaccalaureate Student
We investigate the molecular mechanisms by which transmembrane proteins (referred to as cargo) are sorted to different compartments of the endo-membrane system in eukaryotic cells. The system consists of an array of membrane-enclosed organelles, including the endoplasmic reticulum (ER), the Golgi apparatus, the trans-Golgi network (TGN), endosomes, lysosomes, lysosome-related organelles (LROs) (e.g., melanosomes), and various domains of the plasma membrane in polarized cells (e.g., in epithelial cells and neurons). Transport of cargo between the compartments is mediated by carrier vesicles or tubules, which bud from a donor compartment, translocate through the cytoplasm, and eventually fuse with an acceptor compartment. Work in our laboratory focuses on the molecular machineries that mediate these processes, including (1) sorting signals and adaptor proteins that select cargo proteins for packaging into the transport carriers, (2) microtubule motors that drive movement of the transport carriers and other organelles through the cytoplasm, and (3) tethering factors that promote fusion of the transport carriers to acceptor compartments. We study the machineries in the context of various intracellular transport pathways, including endocytosis, recycling to the plasma membrane, retrograde transport from endosomes to the TGN, biogenesis of lysosomes and LROs, and polarized sorting in epithelial cells and neurons. We apply knowledge gained from this research to the elucidation of protein trafficking diseases such as the Hermansky-Pudlak syndrome (HPS), a pigmentation and bleeding disorder, and the MEDNIK syndrome, a neuro-cutaneous disorder. In addition, we study how the molecular mechanisms of protein transport are exploited by intracellular pathogens such as HIV-1.
Function of the BORC complex in the regulation of lysosome movement
The multiple functions of lysosomes are critically dependent on their ability to move bidirectionally along microtubules between the center and periphery of the cell. Centrifugal and centripetal movement of lysosomes is mediated by kinesin and dynein motors, respectively. We recently discovered a multi-subunit complex named BORC, which recruits the small GTPase Arl8 to lysosomes to promote their kinesin-dependent movement toward the cell periphery. We showed that BORC and Arl8 function upstream of two structurally distinct kinesin types: kinesin-1 (KIF5B) and kinesin-3 (KIF1B and KIF1A). Remarkably, KIF5B and KIF1B/KIF1A move lysosomes along different microtubule tracks. The findings thus established BORC as a master regulator of lysosome positioning through coupling to different kinesins and microtubule tracks.
The BORC complex coordinates encounter and fusion of lysosomes with autophagosomes.
Whereas the mechanisms involved in autophagosome formation have been extensively studied for the past two decades, those responsible for autophagosome-lysosome fusion have only recently begun to garner attention. We recently found that BORC is also required for efficient autophagosome-lysosome fusion. Knock-out (KO) of BORC subunits impairs both the encounter and fusion of autophagosomes with lysosomes. Reduced encounters result from an inability of lysosomes to move toward the peripheral cytoplasm, where many autophagosomes are formed. BORC KO also reduces the recruitment of the HOPS (homotypic fusion and vacuole protein sorting) tethering complex to lysosomes and assembly of a trans–SNARE (SNARE proteins mediate vesicle fusion) complex involved in autophagosome-lysosome fusion. Through these dual roles, BORC integrates the kinesin-dependent movement of lysosomes toward autophagosomes with HOPS–dependent autophagosome-lysosome fusion.
A Ragulator–BORC interaction controls lysosome positioning in response to amino acid availability.
Lysosomes play key roles in the cellular response to amino acid availability. Depletion of amino acids from the medium turns off a signaling pathway involving the Ragulator complex and the Rag GTPases, causing release of the inactive mTORC1 serine/threonine kinase from the lysosomal membrane. Decreased phosphorylation of mTORC1 substrates inhibits protein synthesis while activating autophagy. Amino acid depletion also causes clustering of lysosomes in the juxtanuclear area of the cell, but the mechanisms responsible for this phenomenon are poorly understood. This past year, we showed that Ragulator directly interacts with BORC, the multisubunit complex previously found to promote lysosome dispersal through coupling to the small GTPase Arl8 and the kinesins KIF1B and KIF5B (see above). Interaction with Ragulator exerts a negative regulatory effect on BORC that is independent of mTORC1 activity. Amino acid depletion strengthens this interaction, explaining the redistribution of lysosomes to the juxtanuclear area. The findings demonstrate that amino acid availability controls lysosome positioning through Ragulator-dependent, but mTORC1–independent, modulation of BORC.
Segregation in the Golgi complex precedes export of endo-lysosomal proteins in distinct transport carriers.
Biosynthetic sorting of newly synthesized transmembrane cargos to endosomes and lysosomes is thought to occur at the TGN through recognition of sorting signals in the cytosolic tails of the cargos by adaptor proteins, leading to cargo packaging into coated vesicles destined for the endo-lysosomal system. This past year, we obtained evidence for a different mechanism, by which two sets of endo-lysosomal proteins undergo early segregation to distinct domains of the Golgi complex by virtue of the proteins’ luminal and transmembrane domains. Proteins in one Golgi domain exit into predominantly vesicular carriers by interaction of sorting signals with adaptor proteins, but proteins in the other domain exit into predominantly tubular carriers shared with plasma membrane proteins, independently of signal-adaptor interactions. The findings demonstrate that sorting of endo-lysosomal proteins begins at an earlier stage than, and involves mechanisms that differ from, those described by classical models.
Polarized organelle segregation in neurons by differential interactions with microtubule motors
Polarized sorting of newly synthesized proteins to the somato-dendritic and axonal domains of neurons occurs by selective incorporation into distinct populations of vesicular transport carriers. We recently found that segregation of these carriers to their corresponding neuronal compartments occurs at a region in the axon hillock named the pre-axonal exclusion zone (PAEZ) though differential coupling to different microtubule motors. We also discovered a chain of interactors including Rab5, the FHF complex, and dynein-dynactin, which retrieve somatodendritic proteins from the axon, thus contributing to their somatodendritic distribution at steady state. We further demonstrated that an ensemble composed of BORC, Arl8, SKIP, and kinesin-1 specifically drives lysosome transport into the axon, and not the dendrites, in cultured rat hippocampal neurons. We also found that the transport is essential for the maintenance of axonal growth-cone dynamics and autophagosome turnover. The findings illustrate how a general mechanism for lysosome dispersal in non-neuronal cells is adapted to drive polarized transport in neurons and, furthermore, emphasize the importance of this mechanism for critical axonal processes.
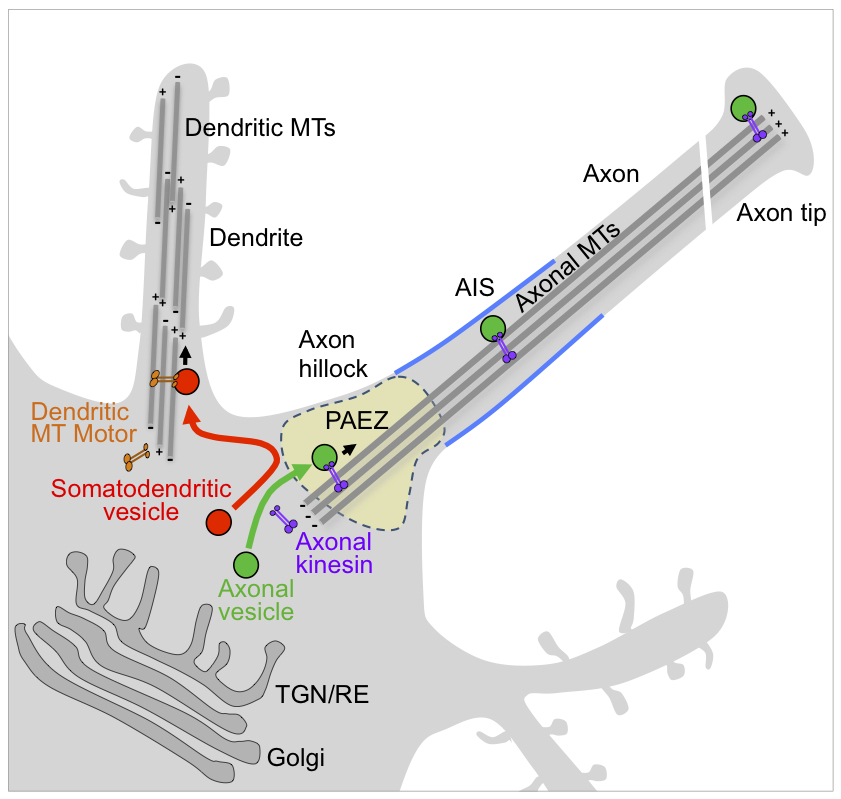
Click image to enlarge.
Figure 1. Sorting of somato-dendritic and axonal vesicles at the pre-axonal exclusion zone (PAEZ)
A role for AP-1 in sorting presenilin-2 to late endosomes and lysosomes
In collaboration with Wim Annaert, we demonstrated a role for the AP-1 complex in the sorting of a novel form of gamma-secretase to late endosomes and lysosomes. Gamma-secretases are a family of intramembrane-cleaving proteases involved in the pathogenesis of Alzheimer’s disease. We identified a sorting motif in the cytosolic tail of the presenilin-2 (PSEN2) subunit of gamma-secretase that targets this enzyme to late endosomes and lysosomes. The motif is recognized in a phosphorylation-dependent manner by AP-1. PSEN2 selectively cleaves late endosomal/lysosomal-localized substrates and generates a prominent pool of intracellular amyloid-beta peptide. The findings reveal potentially important roles for lysosome-generated amyloid-beta peptide in Alzheimer’s disease.
Mechanism of cargo recognition by the retromer complex
Retromer is a multi-protein complex that recycles transmembrane cargo from endosomes to the TGN and the plasma membrane. Defects in retromer impair various cellular processes and underlie some forms of Alzheimer’s disease and Parkinson's disease. Although retromer was discovered over 15 years ago, the mechanisms for cargo recognition and recruitment to endosomes had remained elusive. X-ray–crystallographic, biochemical, and cellular studies conducted in collaboration with Aitor Hierro showed that cargos bind to a cooperative assembly of the VPS26 and VPS35 subunits of retromer in complex with the sorting nexin SNX3.
Identification of TSSC1 as a novel component of the endosomal retrieval machinery
Endosomes function as a hub for multiple protein sorting events, including retrograde transport to the TGN and recycling to the plasma membrane, processes that are mediated by tubular-vesicular carriers that bud from early endosomes and fuse with a corresponding acceptor compartment. We previously investigated the role of two multisubunit tethering complexes named GARP and EARP, which participate in SNARE–dependent fusion of endosome-derived carriers with the TGN and recycling endosomes, respectively. We have now discovered that a previously uncharacterized WD40/beta-propeller protein named TSSC1 is a specific interactor of both GARP and EARP and a novel component of the endosomal retrieval machinery. Interference with TSSC1 impairs both retrograde transport to the TGN and retrieval to the plasma membrane. The findings contribute to an understanding of the pathogenesis of progressive cerebello-cerebral atrophy type 2, a neurodegenerative disorder caused by mutations in the shared Vps53 subunit of GARP and EARP.
Additional Funding
- Intramural AIDS Targeted Antiviral Program (IATAP)
Publications
- Pu J, Keren-Kaplan T, Bonifacino JS. A ragulator-BORC interaction controls lysosome positioning in response to amino acid availability. J Cell Biol 2017 216(12):4183-4197.
- Chen Y, Gershlick DC, Park SY, Bonifacino JS. Segregation in the Golgi complex precedes export of endolysosomal proteins in distinct transport carriers. J Cell Biol 2017; 216(12):4141-4151.
- Jia R, Guardia CM, Pu J, Chen Y, Bonifacino JS. BORC coordinates encounter and fusion of lysosomes with autophagosomes. Autophagy 2017; 13(10):1648-1663.
- Farías GG, Guardia CM, De Pace R, Britt DJ, Bonifacino JS. BORC/kinesin-1 ensemble drives polarized transport of lysosomes into the axon. Proc Natl Acad Sci USA 2017 114:E2955-E2964.
- Guardia CM, Farías GG, Jia R, Pu J, Bonifacino JS. BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep 2016 15:1950-1961.
Collaborators
- Wim Annaert, PhD, VIB Center for the Biology of Disease, Katholieke Universiteit, Leuven, Belgium
- Aitor Hierro, PhD, CIC-bioGUNE, Bilbao, Spain
Contact
For more information, email bonifacinoj@helix.nih.gov or visit https://irp.nih.gov/pi/juan-bonifacino.


