Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Melanija Tomić, PhD, Staff Scientist
- Aloa L. Dams, PhD, Visiting Fellow
- Marija M. Janjic, PhD, Visiting Fellow
- Rafael M. Previde, MD, Visiting Fellow
- Milos B. Rokic, PhD, Visiting Fellow
- Marko Jovic, PhD, Guest Researcher
- Jovana Tavcar, MD, Special Volunteer
We investigate cellular signaling cascades, gene expression, and hormone secretion in hypothalamic and pituitary cells, with a special emphasis on the interactions between plasma-membrane electrical events and receptor-controlled pathways. Specifically, we address how these neuroendocrine cells use ion channels and G protein–coupled receptors as signaling platforms to efficiently process information. To this end, we characterize both native and recombinant receptors and channels that have been cloned from neuroendocrine cells. In the past, our work has focused on the role of inositol-trisphosphate receptors in the oscillatory calcium release by pituitary cells, the mechanism of periodic activation of these channels, and the complex mode of synchronization of calcium release from intracellular stores with electrical activity of cells. We also characterized voltage-gated channels expressed in neuroendocrine cells, the cell type–specific patterns of electrical activity and channels involved, the physiological relevance of such activity, and the crosstalk between G protein–coupled receptors and ion channels. More recently, we characterized ligand-gated receptor channels expressed in pituitary cells, including the ATP–gated P2X receptor channels. Our current work focuses on age-, sex-, and tissue structure–specific signaling, transcription, and secretion in the pituitary gland, the heterogeneity of secretory pituitary cells reflecting their embryonal and postnatal genesis, and cell type–specific exocytic pathways. We are also studying how the structural features of P2X receptors relate to the channels' functions and how plasma membrane receptors and the intracellular signaling milieu affect channel activity.
Characterization of purinergic P2X receptor channel functions
In collaboration with Ivan Milenkovic, we studied the role of P2X receptor channels in electrical activity of maturing auditory neurons. Using brain-slice recordings before hearing onset and in vivo recordings with iontophoretic drug applications after hearing onset, we showed that cell-specific purinergic modulation follows a precise tonotopic pattern in the ventral cochlear nucleus of developing gerbils. In high-frequency regions, ATP responsiveness diminished before hearing onset. In low-to-mid frequency regions, ATP modulation persisted after hearing onset in a subset of low-frequency bushy cells (characteristic frequency lower than 10 kHz). Down-regulation of P2X2/3R currents along the tonotopic axis occurs simultaneously with an increase in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents, thus suggesting a high-to-low frequency maturation pattern. Facilitated action potential (AP) generation, measured as higher firing frequency, shorter excitatory postsynaptic potential–AP delay in vivo, and shorter spike latency in slice experiments, is consistent with increased synaptic efficacy caused by ATP. By combining recordings and pharmacology in vivo, in slices and in human embryonic kidney 293 cells, we showed that the long-lasting change in intrinsic neuronal excitability is mediated by the P2X2/3R (Reference 1).
In collaboration with Anmar Khadra, we also studied allosteric regulation of P2X4 channels by ivermectin (IVM). In general, treatment with IVM increases the efficacy of ATP to activate the P2X4 channel, slows both receptor desensitization during sustained ATP application and receptor deactivation after ATP washout, and makes the channel pore permeable to NMDG+, a large organic cation. Previously, we developed a Markov model based on the presence of one IVM binding site, which described some effects of this compound on rat P2X4 channels. Recently, we presented two novel models, both with three IVM binding sites. The simpler one-layer model can reproduce many of the observed time series of evoked currents but does not capture well the short time scales of activation, desensitization, and deactivation. A more complex two-layer model can reproduce the transient changes in desensitization observed upon IVM application, the significant increase in ATP–induced current amplitudes at low IVM concentrations, and the modest increase in the unitary conductance. In addition, the two-layer model suggests that this receptor can exist in a deeply inactivated state, not responsive to ATP, and that its desensitization rate can be altered by each of the three IVM binding sites. In summary, the study provides a detailed analysis of P2X4 receptor channel kinetics and elucidates the orthosteric and allosteric mechanisms regulating its channel gating (Reference 2).
The cyclin-dependent kinase 5 (Cdk5) plays key roles in brain development and function, including neuronal migration, membrane transport, axon guidance, and pain signaling. Earlier, we had reported that Cdk5 phosphorylates the transient receptor potential vanilloid 1, a key ion channel implicated in pain, increasing its function. In collaboration with Claudio Coddou, we thus focused on the potential role of a putative Cdk5 phosphorylation site in the full-size variant P2X2a receptor channel, which is absent from the splice variant P2X2b channel. We found an interaction between P2X2a and Cdk5/p35 by co-immunofluorescence and co-immunoprecipitation in HEK293 cells. We also found that threonine phosphorylation was significantly higher in HEK293 cells co-expressing P2X2a and p35 than in cells expressing only P2X2a channels. Moreover, P2X2a–derived peptides encompassing the Cdk5 consensus motif were phosphorylated by Cdk5/p35. Whole-cell patch-clamp recordings indicated a delay in development of use-dependent desensitization (UDD) of P2X2a but not of P2X2b receptor in HEK293 cells co-expressing these receptors and p35. In Xenopus oocytes, P2X2a receptors showed a slower UDD than in HEK293 cells and Cdk5 activation prevented this effect. A similar effect was found in P2X2a/3R heteromeric currents in HEK293 cells. The P2X2a-T372A receptor mutant was resistant to UDD. In endogenous cells, we observed similar distribution between P2X2a receptor and Cdk5/p35 by co-localization using immunofluorescence in primary culture of nociceptive neurons. Moreover, co-immunoprecipitation experiments showed an interaction between Cdk5 and P2X2a receptor in mouse trigeminal ganglia. Endogenous P2X2a receptor–mediated currents in PC12 cells and P2X2/3 channel–mediated increases of intracellular calcium in trigeminal neurons were Cdk5–dependent, given that inhibition with the cyclin-dependent kinase inhibitor roscovitine accelerated the desensitization kinetics of these responses. The results indicate that the P2X2a receptor is a novel target for Cdk5–mediated phosphorylation, which might play important physiological roles including pain signaling (Reference 3).
We also studied interactions of pannexin1 channels with purinergic and N-methyl-D-aspartate (NMDA) receptor channels. Pannexins are a three-member family of vertebrate plasma membrane–spanning molecules that have homology to innexins, the invertebrate gap junction–forming proteins. However, pannexins do not form gap junctions but operate as plasma membrane channels. It has been suggested that the best characterized member of this protein family, Pannexin1 (Panx1), is functionally associated with purinergic P2X and NMDA receptor channels. Activation of these receptor channels by their endogenous ligands leads to cross-activation of Panx1 channels, which in turn potentiates P2X and NMDA receptor–channel signaling. Two potentiation concepts have been suggested: enhancement of the current responses and/or sustained receptor-channel activation by ATP released through the Panx1 pore and adenosine generated by ectonucleotidase-dependent dephosphorylation of ATP. Figure 1 summarizes the current knowledge and our hypotheses about interactions of Panx1 channels with P2X and NMDA receptor channels.
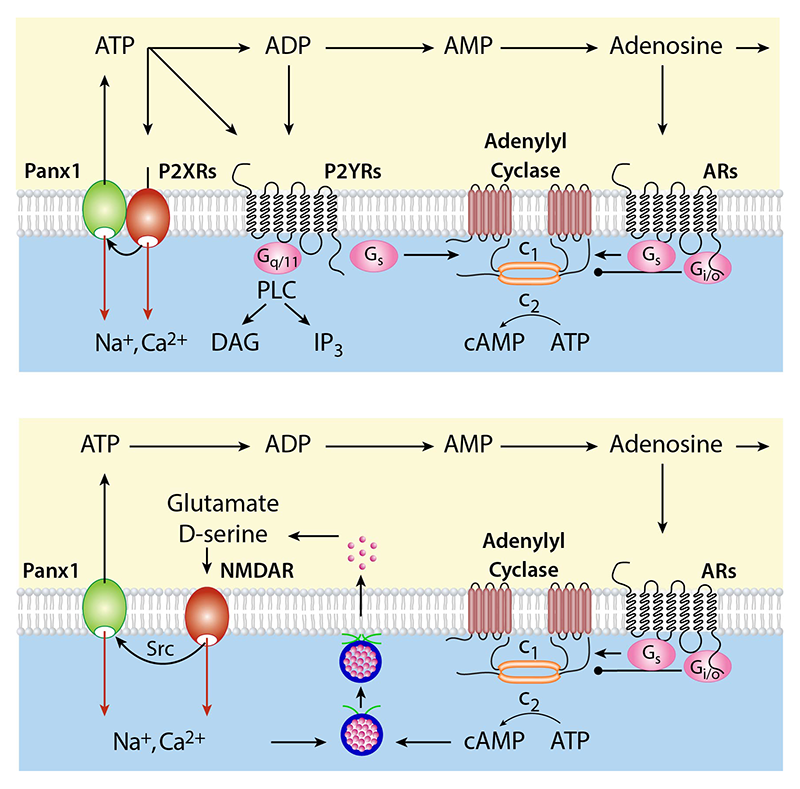
Click image to enlarge.
Figure 1. The crosstalk between Panx1 channels and ligand-gated receptor channels
Top panel: Interactions between Panx1 channels and P2X receptors (P2XRs). Activated P2XRs cross-activate Panx1 channels probably through the Src family kinases. This leads to potentiation of P2XR signaling. Two pathways have been proposed: enhancement of the current (red arrows) and continued activation of P2XRs and co-activation of P2YRs, and ARs (black arrows). Opening of the Panx1 channel facilitates ATP release, which acts as agonist at P2XRs and P2YRs before ectonucleotidases metabolize it into ADP, AMP, and adenosine. ADP also acts as natural ligand for some P2YRs, whereas adenosine is natural ligand for ARs. P2YRs signals through Gq/11 and Gs signaling pathway, whereas ARs facilitate adenylyl cyclase activity through Gs coupling and inhibit this enzyme through Gi/o coupling. Both autocrine and paracrine modes of action were reported.
Bottom panel: Interactions between Panx1 channels and N-methyl-D-aspartate receptor (NMDAR) channels. Activated NMDAR cross-activates Panx1 through the Src family kinases, which in turn potentiate the NMDAR signaling, by enhancement of the current (red arrows) and/or through ATP released from Panx1 and ATP's dephosphorylation to adenosine, which binds to adenosine receptors to diminish neuronal excitability (Gi/o coupling) or provides sustained NMDR activity, probably through release of glutamate and D-serine (Gs-coupling). ARs, adenosine receptors; P2XRs, ATP–gated purinergic P2X receptor channels; P2YRs, purinergic G protein–coupled P2Y receptors; PLC, phospholipase C; DAG diacylglycerol; IP3, inositol (1,4,5) trisphosphate; NMDAR, N-methyl-D-aspartate receptor.
Electrophysiological properties of secretory pituitary cells
Recently, we summarized our investigations on electrical properties and calcium signaling in pituitary gonadotrophs, which are basophilic cells of the anterior pituitary gland specialized to secrete gonadotropins in response to elevation in intracellular calcium concentration. The cells fire APs spontaneously, coupled with voltage-gated calcium influx of insufficient amplitude to trigger gonadotropin release. The spontaneous excitability of gonadotrophs reflects the expression of voltage-gated sodium, calcium, potassium, non-selective cation-conducting, and chloride channels at their plasma membrane. The cells also express the hyperpolarization-activated and cyclic nucleotide–gated cation channels at the plasma membrane, as well as the GABA receptor γ-aminobutyric acid-A, nicotinic, and purinergic P2X receptor channels gated by GABA, acetylcholine, and ATP, respectively. Activation of the channels leads to initiation or amplification of the pacemaking activity, facilitation of calcium influx, and activation of the exocytic pathway. Gonadotrophs also express calcium-conducting channels at the endoplasmic reticulum membranes gated by inositol trisphosphate and intracellular calcium. These channels are activated potently by hypothalamic gonadotropin-releasing hormone (GnRH) and less potently by several paracrine calcium-mobilizing agonists, including pituitary adenylate cyclase–activating peptides, endothelins, acetylcholine, vasopressin, and oxytocin. Activation of the channels causes oscillatory calcium release and a rapid gonadotropin release, accompanied by a shift from tonic firing of single APs to periodic bursting type of electrical activity, which accounts for sustained calcium signaling and gonadotropin secretion (Reference 4).
Our collaborative work with Arthur Sherman focused on modeling the diversity of spontaneous and agonist-induced electrical activity in cultured corticotrophs, the anterior pituitary cell type critical in stress response. These cells fire APs spontaneously and in response to stimulation with corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), and such electrical activity is critical for calcium signaling and calcium-dependent adrenocorticotropic hormone secretion. The cells typically fire tall, sharp APs when spontaneously active, but a variety of other spontaneous patterns have also been reported, including various modes of bursting. Reports vary as to the fraction of corticotrophs that are electrically active, as well as their patterns of activity, and the sources of this variation are not well understood. The ionic mechanisms responsible for CRH– and AVP–triggered electrical activity in corticotrophs are also poorly characterized. We use electrophysiological measurements in single cultured corticotrophs and mathematical modeling to investigate possible sources of variability in patterns of spontaneous and agonist-induced electrical activity. In the model, variation in as few as two parameters can give rise to many of the types of patterns observed in electrophysiological recordings of corticotrophs. We compared the known mechanisms for CRH, AVP, and glucocorticoid action and found that various ionic mechanisms can contribute in different but complementary ways to generate the complex time courses of CRH and AVP responses. In summary, our modeling suggests that corticotrophs have several mechanisms at their disposal to achieve their primary function of pacemaking depolarization and increased electrical activity in response to CRH and AVP. The finding is consistent with critical roles of electrical activity in function of these cells (Reference 5).
In collaboration with the same group, we also examined common and diverse elements of ion channels and receptors underling electrical activity in six major secretory pituitary cells: corticotrophs, melanotrophs, gonadotrophs, thyrotrophs, somatotrophs, and lactotrophs. All these cell types are electrically excitable, and voltage-gated calcium influx is the major trigger for their hormone secretion. Along with hormone intracellular content, G protein–coupled receptor and ion channel expression can also be considered as defining cell-type identity. While many aspects of the developmental and activity-dependent regulation of hormone and G protein–coupled receptor expression have been elucidated, much less is known about the regulation of the ion channels needed for excitation-secretion coupling in these cells. We compare the spontaneous and receptor-controlled patterns of electrical signaling among endocrine pituitary cell types, including insights gained from mathematical modeling. We argue that a common set of ionic currents unites these cells, while differential expression of another subset of ionic currents could underlie cell type–specific patterns. We supported these ideas using a generic mathematical model, showing that it reproduces many of the observed features of pituitary electrical signaling. Mapping these observations to the developmental lineage suggests possible modes of regulation that may give rise to mature pituitary cell types.
Publications
- Jovanovic A, Radulovic T, Coddou C, Dietz B, Nerlich J, Stojilkovic SS, Rubsamen, Milenkovic I. Tonotopic action potential tuning of maturing auditory neurons through endogenous ATP. J Physiol 2017 595:1315-1337.
- Mackay L, Zemkova H, Stojilkovic SS, Sherman A, Khadra A. Deciphering the regulation of P2X4 receptor channel gating by ivermectin using Markov models. PloS Comput Biol 2017 13(7):e1005643.
- Coddou C, Sandoval R, Castro P, Lazcano P, Hevia MJ, Rokic M, Hall B, Terse A, Gonzalez-Billault C, Kulkarni AB, Stojilkovic SS, Utreras E. Cyclin-dependent kinase 5 modulates the P2X2a receptor channel gating through phosphorylation of C-terminal threonine 372. Pain 2017 158:2155-2168.
- Stojilkovic SS, Bjelobaba I, Zemkova H. Ion channels of pituitary gonadotrophs and their roles in signaling and secretion. Front Endocrinol (Lausanne) 2017 8:126:10.3389.
- Fletcher PA, Zemkova H, Stojilkovic SS, Sherman A. Modeling the diversity of spontaneous and agonist-induced electrical activity in anterior pituitary corticotrophs. J Neurophysiol 2017 117:2298-2311.
Collaborators
- Claudio Coddou, PhD, Faculty of Medicine, Universidad Católica del Norte, Coquimbo, Chile
- Anmar Khadra, PhD, McGill University, Montreal, Canada
- Ivan Milenkovic, PhD, Universität Leipzig, Leipzig, Germany
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Hana Zemková, PhD, Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic
Contact
For more information, email stankos@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx or irp.nih.gov/pi/stanko-stojilkovic.


