Childhood Neurodegenerative Lysosomal Storage Disorders

- Anil B. Mukherjee, MD, PhD, Head, Section on Developmental Genetics
- Maria B. Bagh, PhD, Research Fellow
- Abhilash Appu, PhD, Visiting Fellow
- Avisek Mondal, PhD, Visiting Fellow
- Tamal Sadhukhan, PhD, Visiting Fellow
- Sondra W. Levin, MD, Adjunct Scientist
- Zhongjian (Gary) Zhang, MD, PhD, Adjunct Scientist
- Sydney Casey, AB, Postbaccalaureate Intramural Research Training Award Fellow
We conduct both laboratory and clinical investigations into a group of the most common childhood neuro-degenerative lysosomal storage disorders (LSDs), called neuronal ceroid lipofuscinoses (NCLs) and commonly known as Batten disease. The diseases affect mostly children. There is no effective treatment for any of the NCLs. Mutations in over 13 different genes (called the CLNs) underlie various types of NCLs. Among these genes, CLN1, CLN2, CLN10, and CLN13 encode soluble lysosomal enzymes; CLN4 and CLN14 encode peripherally associated cytoplasmic proteins; CLN5 encodes a soluble lysosomal protein; CLN11 encodes progranulin, a protein in the secretory pathway; and several transmembrane proteins with varying subcellular localizations are encoded by CLN3, CLN6, CLN7, CLN8, and CLN12.
Currently, our research focuses on understanding the molecular mechanisms of pathogenesis underlying infantile NCL (INCL: CLN1-disease), juvenile NCL (JNCL: CLN3-disease), and congenital NCL (CNCL: CLN10-disease). Interestingly, all 14 NCL types share some common pathologic features such as intracellular accumulation of autofluorescent material, epileptic seizures, progressive psychomotor decline resulting predominantly from loss of cortical neurons in the cerebrum, neuro-inflammatory findings, visual impairment resulting from retinal degeneration, and shortened lifespan. We first started investigating the INCL (CLN1-disease), which is caused by mutations in the CLN1 gene encoding a lysosomal depalmitoylating enzyme, palmitoyl-protein thioesterase-1 (PPT1). Numerous proteins in the body, especially in the brain, undergo post-translational modification called S-palmitoylation (also called S-acylation). In this process, a long-chain fatty acid is attached to specific cysteine residues in polypeptides via thioester linkage. While S-palmitoylation plays important roles in membrane anchorage of soluble proteins, protein-protein interaction, and protein stability, these proteins must also be depalmitoylated for recycling or degradation in lysosome. PPT1 catalyzes the cleavage of thioester linkage S-palmitoylated proteins. This is important because S-palmitoylated proteins are refractory to degradation by lysosomal hydrolases, and PPT1 deficiency leads to lysosomal accumulation of these lipidated proteins (constituents of ceroid), leading to the pathogenesis of INCL. Children afflicted with INCL are normal at birth but, by 11 to 18 months of age, they exhibit signs of psychomotor retardation. By 2 years of age, they are completely blind owing to retinal degeneration and, by age 4, they manifest no brain activity and remain in a vegetative state for 6 to 8 more years before eventual death. Such grim outcomes underscore the urgent need for the development of rational and effective therapeutic strategies not only for INCL but also for all NCLs.
The aim of our clinical studies is to apply the knowledge gained from laboratory investigations to develop novel therapeutic strategies for Batten disease. The results of our earlier investigations on INCL (CLN1-disease) led to a bench-to-bedside clinical trial (Reference 5). Using Cln1-knockout (Cln1–/–) mice, which recapitulate virtually all clinical and pathological features of INCL, we discovered that PPT1 deficiency causes endoplasmic reticulum (ER) and oxidative stress, which at least in part causes neuronal death by apoptosis. During the past several years, we also delineated a mechanism by which PPT1 deficiency disrupts the recycling of the synaptic vesicle (SV) proteins, which are essential for regenerating fresh SVs to replenish the SV pool size at the nerve terminals to maintain uninterrupted neurotransmission. We also discovered that ER and oxidative stress contribute to neuronal apoptosis and neuro-inflammation in INCL. Further, we found that PPT1 deficiency causes misrouting of the V0a1 subunit of v-ATPase (the proton pump on lysosomal membrane), which regulates lysosomal acidic pH, causing elevated pH, which adversely affects lysosomal degradative function (Reference 1).
We also developed a non-invasive methods, using MRI and MRS (magnetic resonance spectroscopy), to evaluate the progression of neuro-degeneration in Ppt1–/– mice. These methods permit repeated evaluations of potential therapeutic agents in treated animals. Application of the methods in our clinical trial with INCL also allowed us to evaluate the progressive decline in brain volume and neuro-degeneration (Reference 2). In addition, in collaboration with the NEI, we are conducting studies to determine whether electro-retinography can be used to assess the progressive retinal deterioration in Cln1–/– as well as in Cln1–knock-in (KI) mice generated in our laboratory, which carry the most common nonsense mutation found in the INCL patient population in the US. We also discovered that the blood-brain barrier is disrupted in Ppt1–/– mice and that the pathology is ameliorated by treatment with resveratrol, which has anti-oxidant properties. More recently, we discovered that a nucleophilic small molecule with antioxidant properties, N-(tert-butyl) hydroxylamine (NtBuHA), ameliorates the neurological abnormalities in Cln1–/– mice and extends their lifespan (Reference 4). These and related studies provide insight into the complex mechanisms of heritable disorders of neuro-degeneration like INCL (CLN1-disease) and identify several potential therapeutic targets. Our results suggest that thioesterase-mimetic small molecules such as NtBuHA are potential therapeutic targets for INCL. More recently, we discovered that cathepsin D (CD) deficiency in lysosomes is a common pathogenic link between INCL (CLN1 disease) and congenital NCL (CNCL) or CLN10 disease. Our ongoing laboratory and clinical investigations are attempting to advance our knowledge of CLN1, CLN3, and CLN10 diseases. Our long-term plans are to apply the new findings arising from our laboratory studies to discover the pathogenic links among various NCLs and to develop novel therapeutic strategies not only for CLN1 disease but also for CLN3 and CLN10 diseases.

Click image to enlarge.
Section on Developmental Genetics Staff
Upper Left to Right: Sydney Casey, Abhilash Appu, Tamal Sadhukhan, and Maria Bagh.
Lower Left to Right: Sondra Levin, Gary Zhang, and Anil Mukherjee
Dysregulation of lysosomal acidification in the INCL mouse model
In eukaryotic organisms, the lysosome is the primary organelle for intracellular digestion. It contains more than 50 hydrolases, which require an acidic pH for optimal degradative function. Thus, lysosomal acidification is of fundamental importance in the degradation of macromolecules of intra- and extra-cellular origin that are delivered to the lysosome. Moreover, it has been reported that dysregulation of lysosomal acidification contributes to pathogenesis in virtually all lysosomal storage disorders (LSDs), including several NCLs. Furthermore, defective regulation of lysosomal pH has also been reported in common neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. However, despite intense studies, the mechanism(s) underlying the lysosomal acidification defect remains largely unclear. Lysosomal acidification is regulated by vacuolar ATPase (v-ATPase), a multisubunit protein complex composed of the cytosolic V1 sector and the lysosomal membrane–anchored V0-sector. Reversible assembly of V1/V0 sectors on the lysosomal membrane maintains functionally active v-ATPase, the proton pump of the cell
In the mammalian genome, 23 genes encode palmitoyl-acyl-transferases (PATs), which are evolutionarily conserved, cysteine-rich proteins containing Asp-His-His-Cys (DHHC) in the active site. In contrast, there are four thioesterases that have been characterized thus far. Two of these are cytosolic (acyl-protein thioesterase-1 [Apt1] and Apt2) and two (palmitoyl-protein thioesterase-1 [PPT1] and PPT2) are localized to the lysosome. Dynamic palmitoylation (palmitoylation-depalmitoylation), requiring coordinated action of both the DHHC-PATs and PPTs, maintains steady-state membrane localization and the function of numerous important proteins, especially in the brain. By catalyzing depalmitoylation, thioesterases also facilitate recycling or degradation of S-palmitoylated proteins (constituents of ceroid) by lysosomal hydrolases.
We tested the hypothesis that one or more subunits of v-ATPase may requires S-palmitoylation for endosomal sorting, trafficking, and reversible assembly of V1/V0 on the lysosomal membrane, which is essential for regulating lysosomal pH, and that Ppt1 deficiency disrupts v-ATPase activity, impairing its proton transport function, thereby dysregulating acidification of lysosomal lumen. Our results show that the lysosomal membrane–anchored V0a1 subunit of v-ATPase undergoes S-palmitoylation, which is required for its sorting and trafficking to the lysosomal membrane. The process appears to be defective in Ppt1–deficient Cln1–/– mice. Notably, we demonstrated that treatment of these mice with the thioesterase (Ppt1)–mimetic small molecule NtBuHA restores v-ATPase activity and rescues the defective lysosomal acidification phenotype.
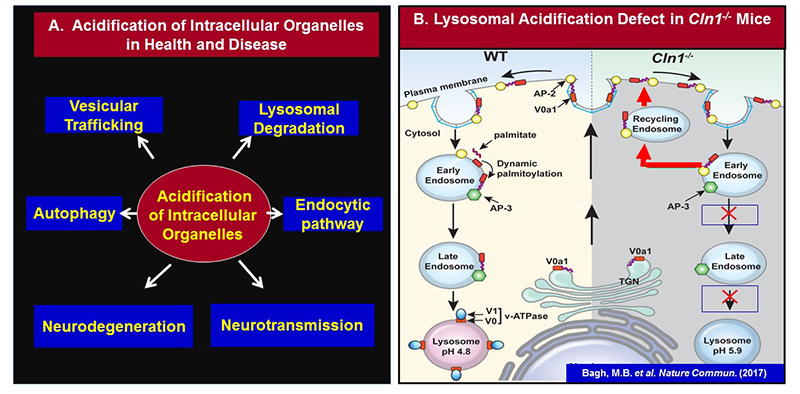
Click image to enlarge.
Figure 1.
A. Importance of acidification of intracellular organelles in health and disease
B. Schematic representation of endosomal sorting and trafficking of V0a1 in WT (left panel) and in Cln1-/- (right panel) mice.V0a1 fails to dissociate from AP-2 in Cln1-/- cells, preventing it from interacting with AP-3, required for its transport from the sorting endosome to the late endosomal/lysosomal membrane. Consequently, the V0a1–AP-2 complex is misrouted via recycling endosome to the plasma membrane. This defect impairs lysosomal v-ATPase activity, thereby dysregulating lysosomal acidification in neurons of Cln1-/- mice, which mimic INCL.
Evaluation of INCL (CLN1) disease progression by magnetic resonance spectroscopy
As stated in the introduction, the accumulation of ceroid is a characteristic pathological finding in all NCLs. Although the components and ultrastructure of the storage material vary across the different types of NCLs, electron-microscopic analyses of the brain and other tissues from INCL patients show characteristic granular osmiophilic deposits (GRODs). Previously, studies using cell cultures derived from INCL patients demonstrated that phosphocysteamine suppresses apoptosis and depletes intra-lysosomal ceroid deposits. N-acetylcysteine, a potent antioxidant, has also been reported to have beneficial effects in other neurodegenerative diseases. Because INCL is a rare disease (1 in over 100,000 births) with a short life expectancy, recruitment of a large study group is impractical. This consideration, together with promising results from cell-culture experiments, led to a “compassionate use” type of experimental design in which all patients received treatment with cysteamine bitartrate (Cystagon) and N-acetylcysteine (Mucomyst), without a control group, with the intention of comparing to the natural history with future treatment interventions, using quantifiable measures. These included behavioral and developmental assessments, EEG, ERG, MRI–derived brain volume measurements, and quantification of intra-lysosomal ceroid deposits. Towards the end of the study's recruitment period, quantitative magnetic resonance spectroscopy (MRS) was added to the quantifiable measures. An overview of the study results was published (Reference 5), and details the quantitative MRS findings are listed below.
The evaluation of any treatment benefits of cysteamine bitartrate and N-acetylcysteine included quantitative measurement of brain metabolite levels using MRS. A subset of two patients from a larger treatment and follow-up study underwent serial quantitative single-voxel MRS examinations of five anatomical sites. Three echo times were acquired in order to estimate metabolite T2 (quantification of the absolute concentration of metabolites using long-echo-time [TE] acquisition schemes). Measured metabolite levels included a correction for the partial volume of cerebrospinal fluid. We compared INCL patients with a reference group of asymptomatic and minimally symptomatic Niemann-Pick disease type C patients. In INCL patients, N-acetylaspartate (NAA) was abnormally low at all locations upon initial measurement and further declined throughout the follow-up period. In the cerebrum (affected early in the disease course) choline and myo-inositol levels were initially elevated and fell during the follow-up period, whereas in the cerebellum and brainstem (affected later) choline and myo-inositol levels were initially normal and rose subsequently. Choline and myo-inositol levels in our patients are consistent with patterns of neuro-inflammation observed in two INCL mouse models. Low, persistently declining NAA was expected based on the progressive, irreversible nature of the disease. Progression of metabolite levels in INCL has not been previously quantified; therefore the results of this study serve as a reference for quantitative evaluation of future therapeutic interventions.
Common pathogenic link between INCL (CLN1-disease) and CNCL (CLN10-disease)
The lysosome is the major degradative organelle responsible for disposing of the damaged macromolecules and organelles brought into the cell from external and internal sources. It has been reported that impaired lysosomal degradative capability leads to pathogenesis of many neurodegenerative disorders, including LSDs. Neurodegeneration is a manifestation in the majority of the more than 60 LSDs. Moreover, impaired lysosomal degradative capability has been reported in several late-onset neurodegenerative diseases such as Alzheimer’s, Huntington’s, and Parkinson’s disease. Cathepsin D (CD) is a major lysosomal aspartic protease essential for degradation of proteins delivered to the lysosome. Lysosomal CD activity catalyzes degradation and clearance of exogenous as well as endogenous macromolecules and damaged organelles delivered to the lysosome. Intracellular accumulation of undegraded long-lived proteins and other macromolecules leads to the pathogenesis of many neurodegenerative disorders. Paradoxically, both CD overexpression and CD deficiency have been reported to underlie neurodegenerative diseases. However, despite intense studies, this paradox has, until now, remained poorly understood.
Whereas inactivating mutations in the CLN1 gene, encoding palmitoyl-protein thioesterase-1 (PPT1), cause INCL, mutations in the CLN10/CTSD gene, encoding CD, underlie CNCL (CLN10-disease). We sought to determine whether there is a pathogenic link between INCL and CNCL. The synthesis of CD occurs in the ER as a pre-propeptide with a molecular mass of about 50 kDa. The cleavage of the leader peptide in the ER generates the 48 kDa precursor of mature-CD (pro-CD). In the Golgi complex, attachment of mannose 6-phosphate to pro-CD facilitates the protein's binding to endosomal/lysosomal sorting receptors. The receptor-ligand complexes then exit the trans-Golgi network in clathrin-coated intermediates and fuse with the endosomal system. The low pH of the late endosomal lumen facilitates dissociation of the receptor-ligand complexes and allows the ligand (i.e., pro-CD) to be delivered to lysosome. The pro-CD then undergoes further proteolytic cleavage by cathepsin B (CB) and cathepsin L (CL), which generate, respectively, the 31 and 14 kDa fragments, non-covalent dimerization of which constitutes the mature, catalytically active CD. We used Cln1–/–/Ppt1–/– mice, which recapitulate virtually all clinical and pathological features of INCL, to test for a pathogenic link between INCL and CNCL. Our results show that, despite Cln10/Ctsd overexpression, defective processing of pro-CD to mature CD in lysosome leads to lysosomal CD deficiency causing neuropathology in INCL. Given that CD deficiency underlies CNCL, we propose that CD deficiency in the lysosome is indeed a common pathogenic link between INCL and CNCL.
Non-invasive brain volume measurements in INCL patients by magnetic resonance spectroscopy
Evaluation of the benefits from a treatment using a combination of cysteamine bitartrate (Cystagon) and N-acetylcysteine (Mucomyst) were carried out using serial measurements of patients’ brain volumes with MR imaging (MRI). Ten patients with infantile neuronal ceroid lipofuscinosis participating in a treatment/follow-up study underwent brain MR imaging that included high-resolution, T1-weighted images. After manual placement of a mask delineating the surface of the brain, a maximum-likelihood classifier was applied to determine total brain volume, further subdivided as cerebrum, cerebellum, brain stem, and thalamus. Patients’ brain volumes were compared with those of a healthy population. Major subdivisions of the brain followed similar trajectories with different timing. The cerebrum demonstrated early, rapid volume loss and may never have been normal postnatally. The thalamus dropped out of the normal range at around six months of age, the cerebellum around two years of age, and the brain stem around three years of age. Rapid cerebral volume loss was expected on the basis of previous qualitative reports. Because our study did not include a non-treatment arm and because progression of brain volumes in infantile neuronal ceroid lipofuscinosis has not been previously quantified, we could not determine whether our intervention had a beneficial effect on brain volumes. However, the level of quantitative detail in this study allows it to serve as a reference for evaluation of future therapeutic interventions.
Publications
- Bagh MB, Peng S, Chandra G, Zhang Z, Singh SP, Pattabiraman N, Liu A, Mukherjee AB. Misrouting of v-ATPase subunit V0a1 dysregulates lysosomal acidification in a neurodegenerative lysosomal storage disease model. Nat Commun 2017 8:14612.
- Baker EH, Levin SW, Zhang Z, Mukherjee AB. MRI brain volume measurements in infantile neuronal ceroid lipofuscinosis. Am J Neuroradiol 2017 38:376-382.
- Chandra G, Bagh MB, Peng S, Saha A, Sarkar C, Moralle M, Zhang Z, Mukherjee AB. Cln1 gene disruption in mice reveals a common pathogenic link between two of the most lethal childhood neurodegenerative lysosomal storage disorders. Hum Mol Genet 2015 14:5416-5432.
- Sarkar C, Chandra G, Peng S, Zhang Z, Liu A, Mukherjee AB. Neuroprotection and lifespan extension in Ppt1(-/-) mice by NtBuHA: therapeutic implications for INCL. Nat Neurosci 2013 16:1608-1617.
- Levin SW, Baker EH, Zein WM, Zhang Z, Quezado ZM, Miao N, Gropman A, Griffin KJ, Bianconi S, Chandra G, Khan OI, Caruso RC, Liu A, Mukherjee AB. Oral cysteamine bitartrate and N-acetylcysteine for patients with infantile neuronal ceroid lipofuscinosis: a pilot study. Lancet Neurol 2014 13:777-787.
Collaborators
- Eva Baker, MD, PhD, Radiology and Imaging Sciences, Clinical Center, NIH, Bethesda, MD
- Chris J. McBain, PhD, Section on Cellular and Synaptic Physiology, NICHD, Bethesda, MD
- Kenneth Pelkey, PhD, Section on Cellular and Synaptic Physiology, NICHD, Bethesda, MD
- Ling-Gang Wu, PhD, Synaptic Transmission Section, NINDS, Bethesda, MD
Contact
For more information, email mukherja@exchange.nih.gov or visit irp.nih.gov/pi/anil-mukherjee.


