Thyroid Hormone Regulation of Vertebrate Postembryonic Development

- Yun-Bo Shi, PhD, Head, Section on Molecular Morphogenesis
- Liezhen Fu, PhD, Staff Scientist
- Nga Luu, MS, Biologist
- Wonho Na, PhD, Visiting Fellow
- Morihiro Okada, PhD, Visiting Fellow
- Julia Rodiger, PhD, Visiting Fellow
- Yuki Shibata, PhD, Visiting Fellow
- Yuta Tanizaki, PhD, Visiting Fellow
- Lingyu Bao, MS, Graduate Student
- Lataijah C. Crawford, BS, Postbaccalaureate Fellow
This laboratory investigates the molecular mechanisms of thyroid hormone (TH) function during post-embryonic development. The principal model is the metamorphosis of Xenopus laevis and X. tropicalis, two closely related species, which offer unique but complementary advantages. The control of this developmental process by TH provides a paradigm to study gene function in postembryonic organ development. During metamorphosis, distinct organs undergo vastly different changes. Some, like the tail, undergo complete resorption, while others, such as the limb, are developed de novo. The majority of larval organs persist through metamorphosis but are dramatically remodeled to function in a frog. For example, tadpole intestine is a simple tubular structure consisting primarily of a single layer of larval epithelial cells. During metamorphosis, it is transformed into an organ with a multiply folded adult epithelium surrounded by elaborate connective tissue and muscles, a process that involves specific larval epithelial cell death and de novo development of the adult epithelial stem cells followed by their proliferation and differentiation. The wealth of knowledge from past research and the ability to manipulate amphibian metamorphosis, both in vivo by using genetic approaches or hormone treatment of whole animals and in vitro in organ cultures, offer an excellent opportunity to (1) study the developmental function of TH receptors (TRs) and the underlying mechanisms in vivo and (2) identify and functionally characterize genes that are critical for organogenesis, particularly for the formation of the adult organ-specific stem cells, during postembryonic development in vertebrates. A major recent focus has been to make use of the TALEN and CRISPR/Cas9 technologies (References 1,2) to knockdown or knockout the endogenous genes for functional analyses.
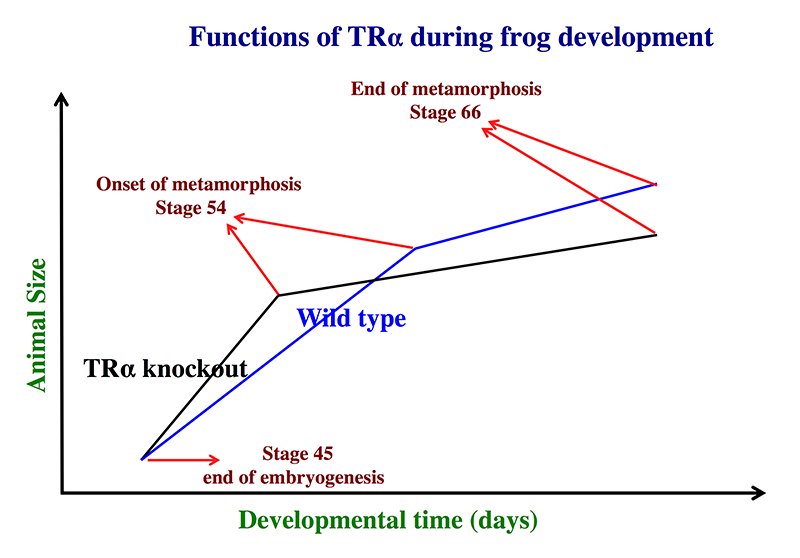
Animals lacking TRα complete metamorphosis around the same age as their wild-type siblings.
Using the TALEN technology, we generated X. tropicalis animals lacking any functional TRα and observed surprisingly that TRα knockout animals are able to complete metamorphosis at a similar age as their wild-typing siblings (Reference 1). Careful analyses during development, however, revealed that the TRα knockout animals initiated metamorphosis at a younger age and with a smaller size but progressed more slowly through metamorphosis. The wild-type siblings initiated metamorphosis at an older age but progressed more quickly, eventually completing metamorphosis around the same time as the knockout animals. As the TRα knockout animals initiated metamorphosis at a smaller size, they were also smaller at the end of metamorphosis. The findings are consistent with our earlier studies with TRα knockdown animals and reveal a critical role of endogenous TRα both in mediating the metamorphic effect of TH during metamorphosis and in preventing precocious initiation of metamorphosis when TH is absent. They thus provide direct evidence to support the dual function model for TR in Xenopus development that we proposed over a decade ago based on gene expression and in vitro function studies.
Click image to enlarge.
Figure 1. Schematics showing the effects of TRα (thyroid hormone α) knockout on Xenopus tropicalis development
TRα knockout has little effect on embryogenesis, and the resulting tadpoles are normal by feeding stage (stage 45). Once feeding begins, the animals grow at different rates, with the knockouts growing faster; they are thus larger than to wild-type siblings at the same age (in days) (comparing the vertical axis values of the lines for the knockout and wild-type animals at any given position along the horizontal axis between stages 45 and 54). The knockout animals also develop faster, reaching developmentally more advanced stages than wild-type siblings at the same age (in days). Thus, the knockout animals reach stage 54, the onset of metamorphosis, at a younger age (see the horizontal axis locations for the upper end of the lines). Interestingly, when the animals are compared at stage 54, the wild-type are larger than the knockout siblings, even though the latter grow faster. This is because the wild-type animals take longer to reach metamorphosis (stage 54). The extra growth time needed to reach stage 54 enables the wild-types to catch up and surpass the knockouts in size. After the initiation of metamorphosis at stage 54, the knockout tadpoles metamorphose more slowly than the wild-type ones, enabling the latter to catch up in development, with both groups finishing metamorphosis at around the same age. The knockout animals initiate metamorphosis at a smaller size and also end up with a smaller size at the end of metamorphosis than do the wild-type siblings. Thus, in premetamorphic tadpoles prior to stage 54, unliganded TRα (due to the lack of thyroid hormone) functions to control metamorphic timing, whereas, when thyroid hormone becomes available during metamorphosis, TRα helps increase the rate of metamorphosis.
Direct transcriptional regulation of the histidine ammonia-lyase 2 gene by the thyroid hormone receptor in developing adult intestinal stem cells
Our early studies in Xenopus laevis showed that intestinal remodeling involves complete degeneration of the larval epithelium and de novo formation of adult stem cells through de-differentiation of some larval epithelial cells. We further discovered that the histidine ammonia-lyase (HAL, also known as histidase or histidinase)-2 gene is strongly and specifically activated by TH in the proliferating adult stem cells of the intestine during metamorphosis, implicating a role of histidine catabolism in the development of adult intestinal stem cells. To determine the mechanism by which TH regulates the HAL2 gene, we carried out a bioinformatics analysis and discovered a putative TH response element (TRE) in the HAL2 gene (Reference 3). Importantly, we showed that this TRE is bound by TH receptor (TR) in the intestine during metamorphosis. The TRE is capable of binding to the heterodimer of TR and 9-cis retinoic acid receptor (RXR) in vitro and mediating transcriptional activation by liganded TR/RXR in frog oocytes. More importantly, the HAL2 promoter containing the TRE can drive TH–dependent reporter gene expression to mimic endogenous HAL2 expression in transgenic animals. Our results suggest that the TRE mediates the transcriptional activation of the HAL2 gene by TH in the developing adult intestinal stem cells during metamorphosis.
A balance of Mad and Myc expression dictates larval cell apoptosis and adult stem cell development during Xenopus intestinal metamorphosis.
The Myc/Mad/Max network has long been known to be an important player in regulating cell proliferation, death and differentiation in diverse cell types. In general, Myc-Max heterodimers activate target gene expression to promote cell proliferation, although excess of c-Myc can also induce apoptosis. In contrast, Mad competes against Myc to form Mad-Max heterodimers that bind to the same target genes to repress their expression and promote differentiation. The role of the Myc/Mad/Max network during vertebrate development, particularly so-called post-embryonic development, a period around birth in mammals, is unclear. We discovered that Mad1 is induced by TH in the intestine during metamorphosis when larval epithelial cell death and adult epithelial stem cell development take place (Reference 4). More importantly, we demonstrated that Mad1 is expressed in larval cells undergoing apoptosis while c-Myc is expressed in proliferating adult stem cells during intestinal metamorphosis, suggesting that Mad1 plays a role in cell death during development. By using the TALEN–mediated gene-editing technology, we generated Mad1 knockout Xenopus animals, revealing that Mad1 is not essential for embryogenesis or metamorphosis. On the other hand, consistent with its spatio-temporal expression profile, Mad1 knockout leads to reduced larval epithelial apoptosis but, surprisingly, also results in increased adult stem cell proliferation. The findings not only reveal a novel role of Mad1 in regulating developmental cell death but also suggest that a balance between Mad and Myc controls cell fate determination during adult organ development.
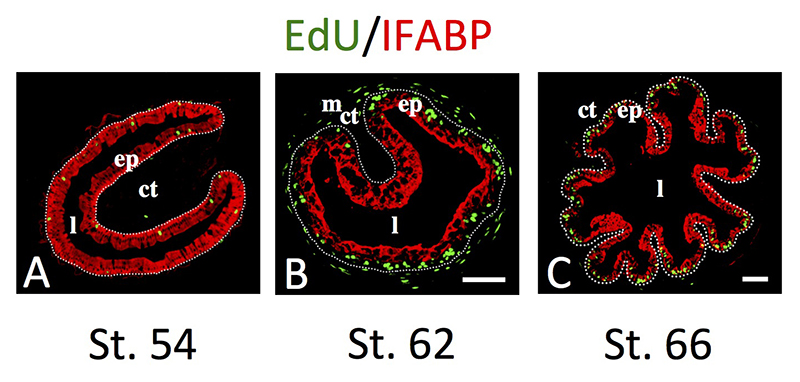
Click image to enlarge.
Figure 2. Intestinal metamorphosis involves the formation of clusters of proliferating, undifferentiated epithelial cells at the climax.
Tadpoles at premetamorphic stage 54 (A), climax (B, stage 62), and end of metamorphosis (C, stage 66) were injected with 5-ethynyl-2′-deoxyuridine (EdU) one hour before being sacrificed. Cross-sections of the intestine from the resulting tadpoles were double-stained by EdU labeling of newly synthesized DNA and by immunohistochemistry of IFABP (intestinal fatty acid binding protein), a marker for differentiated epithelial cells. The dotted lines depict the epithelium-mesenchyme boundary. Note that there are few EdU–labeled proliferating cells in the epithelium and that they express IFABP at premetamorphosis (A) and increase in the form of clustered cells (proliferating adult stem cells) that lack IFABP at the climax of metamorphosis (B). At the end of metamorphosis, EdU–labeled proliferating cells are localized mainly in the troughs of the epithelial folds, where IFABP expression is low (C). ep, epithelium; ct, connective tissue; m, muscles; l, lumen.
Requirement for thyroid hormone–induced activation of Notch signaling for adult intestinal stem cell development during metamorphosis
We showed earlier that TH–induced intestinal remodeling involves regulation of various genes including Notch receptor. To study the role of Notch signaling pathway, we used real-time RT-PCR and in situ hybridization or immunohistochemistry to analyze the expression of various components of this pathway, including the ligands DLL and Jag, the Notch receptor, and targets such as Hairy genes, in the metamorphosing intestine (Reference 5). We showed that they are up-regulated during both natural and TH–induced metamorphosis in a tissue-specific manner. Particularly, Hairy1 is specifically expressed in the adult epithelial stem cells. Moreover, up-regulation of Hairy1 and Hairy2b by TH was prevented by treating tadpoles with a γ-secretase inhibitor (GSI), which inhibits Notch signaling. More importantly, TH–induced up-regulation of LGR5, an adult intestinal stem cell marker, was suppressed by GSI treatment. Our results suggest that Notch signaling plays a role in stem cell development by regulating the expression of Hairy genes during intestinal remodeling. Furthermore, we demonstrated in organ cultures that prolonged exposure of tadpole intestine to TH plus GSI leads to hyperplasia of secretory cells and a reduction in absorptive cells. Our findings thus provide evidence for an evolutionarily conserved role of Notch signaling in intestinal cell fate determination but more importantly reveal, for the first time, an important role of the Notch pathway in the formation of adult intestinal stem cells during vertebrate development.
Methods development
Simple and efficient methods to visualize and quantify the efficiency of chromosomal mutations from genome editing
Genome editing with designer nucleases such as TALEN and CRISPR/Cas enzymes has broad applications. Delivery of these designer nucleases into organisms induces various genetic mutations including deletions, insertions, and nucleotide substitutions. Characterizing those mutations is critical for evaluating the efficacy and specificity of targeted genome editing. While a number of methods have been developed to identify the mutations, none other than sequencing allow the identification of the most desired mutations, i.e., out-of-frame insertions/deletions that disrupt genes. During our studies on gene editing in Xenopus development, we developed a simple and efficient method to visualize and quantify the efficiency of genomic mutations induced by gene editing (Reference 2). Our approach is based on the expression of a two-color fusion protein in a vector that allows the insertion of the edited region in the genome between the two color moieties. We showed that our approach not only easily identifies developing animals with desired mutations but also efficiently quantifies the mutation rate in vivo. Furthermore, by using LacZα and GFP as the color moieties, our approach can even eliminate the need for a fluorescent microscope, allowing the analysis with simple bright field visualization. Such an approach will greatly simplify screening for effective gene-editing enzymes and identify the desired mutant cells and/or animals.
Efficient, simple, non-invasive procedure for genotyping aquatic and non-aquatic laboratory animals
Various animal models are indispensable in biomedical research. Increasing awareness and regulations have prompted the adaptation of more humane approaches in the use of laboratory animals. With the development of easier and faster methodologies to generate genetically altered animals, convenient and humane methods to genotype these animals are important for research involving such animals. To facilitate genotyping of gene-edited Xenopus animals, we developed skin swabbing as a simple and noninvasive method for extracting genomic DNA from tadpoles and frogs for genotyping. The method is highly reliable and suitable for both immature and adult animals, not only for frogs but for also mice. Our approach thus allows a simpler and more humane approach for genotyping vertebrate animals.
Additional Funding
- Japan Society for the Promotion of Science (JSPS) fellowships for Drs. Morihiro Okada and Yuki Shibata
- Lataijah C. Crawford was an NICHD Developing Talent Scholar.
Publications
- Wen L, Shibata Y, Su D, Fu L, Luu N, Shi Y.B. Thyroid hormone receptor a controls developmental timing and regulates the rate and coordination of tissue specific metamorphosis in Xenopus tropicalis. Endocrinology 2017 158:1985-1998.
- Fu L, Wen L, Luu N, Shi Y-B. A simple and efficient method to visualize and quantify the efficiency of chromosomal mutations from genome editing. Sci Rep 2016 6:35488.
- Luu N, Fu L, Fujimoto K, Shi Y-B. Direct regulation of histidine ammonia-lyase 2 gene by thyroid hormone in the developing adult intestinal stem cells. Endocrinology 2017 158:1022-1033.
- Okada M, Miller TC, Wen L, Shi Y-B. A balance of Mad and Myc expression dictates larval cell apoptosis and adult stem cell development during Xenopus intestinal metamorphosis. Cell Death Dis 2017 8(5):e2787.
- Hasebe T, Fujimoto K, Kajita M, Fu L, Shi Y-B, Ishizuya-Oka A. Thyroid hormone-induced activation of Notch signaling is required for adult intestinal stem cell development during Xenopus laevis metamorphosis. Stem Cells 2017 35(4):1028–1039.
Collaborators
- Kenta Fujimoto, PhD, Nippon Medical School, Tokyo, Japan
- Takashi Hasebe, PhD, Nippon Medical School, Tokyo, Japan
- Atsuko Ishizuya-Oka, PhD, Nippon Medical School, Tokyo, Japan
- Joseph M. Schech, DVM, Research Animal Management Branch, NICHD, Bethesda, MD
Contact
For more information, email shi@helix.nih.gov or visit http://smm.nichd.nih.gov.


