Physiology, Psychology, and Genetics of Obesity

- Jack A. Yanovski, MD, PhD, Head, Section on Growth and Obesity
- Joo Yun Jun, PhD, Postdoctoral Fellow
- Miranda Broadney, MD, MPH, Pediatric Endocrine Training Program Fellow
- Nichole Kelly, PhD, Special Volunteer
- Natasha A. Schvey, PhD, Special Volunteer
- Lisa Shank, MA, Special Volunteer
- Sheila M. Brady, RN, FNP, Nurse Practitioner
- Robin Roberson, MS, Technician
- Anne Claire Grammer, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Shannon Marwitz, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Rim D. Mehari, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Sarah Mi, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Viraj Parikh, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Pooja Patel, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Faizah Shareef, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Andrew Uhlman, BS, Postbaccalaureate Intramural Research Training Award Fellow
The prevalence of overweight and obesity in children and adults has tripled during the past 40 years. The alarming rise in body weight has likely occurred because the current environment affords easy access to calorie-dense foods and requires less voluntary energy expenditure. However, such an environment leads to obesity only in those individuals whose body weight–regulatory systems are not able to control body adiposity with sufficient precision in our high calorie/low activity environment, which suggests there are subgroups in the U.S. with a uniquely high susceptibility to weight gain under the prevailing environmental conditions. Our primary goal is to elucidate the genetic underpinnings of the metabolic and behavioral endo-phenotypes that contribute to the development of obesity in children. Using our unique longitudinal cohorts of children at risk for adult obesity, who have undergone intensive metabolic and behavioral phenotyping, we examine genetic and phenotypic factors predictive of progression to adult obesity in children who are in the ‘pre-obese’ state, allowing characterization of phenotypes unconfounded by the impact of obesity itself. Once they are identified as linked to obesity, we study intensively genetic variants that impair gene function. We expect that these approaches will improve our ability to predict which children are at greatest risk for obesity and its comorbid conditions and will thus lead to more targeted, etiology-based prevention and treatment strategies for pediatric obesity.
Click image to enlarge.
Figure 1. Energy intake studied by using free-access buffet meals of palatable foods
Children homozygous for two polymorphisms in the MC3R gene (Hom/Hom) consumed more at the buffet than heterozygotes (Het/Het) or those with wild-type MC3R (Wt/Wt).
Genetic factors important for childhood body weight regulation
To identify gene variants affecting body composition, we have been examining polymorphisms in genes involved in the leptin signaling pathway. Genes include the leptin receptor (LEPR), FTO (fat mass– and obesity-associated gene), and those encoding proopiomelanocortin (POMC), the melanocortin 3 receptor (MC3R), the melanocortin 4 receptor (MC4R), and brain-derived neurotrophic factor (BDNF). We are currently studying a variant MC3R that is associated with adiposity in children and appears to have functional significance for MC3R signal transduction. Children who were homozygous variant for both C17A and G241A polymorphisms have significantly greater fat mass and higher plasma levels of insulin and leptin than unaffected or heterozygous children and appear to eat more at laboratory test meals (Figure 1). In vitro studies subsequently found that signal transduction and protein expression were significantly lower for the double mutant MC3R. Our ongoing studies attempt to understand the mechanisms by which these sequence alterations may affect body weight. Transgenic ‘knock-in’ mice expressing the human wild-type and human double-mutant MC3R were therefore developed. Using homozygous knock-in mouse models replacing murine Mc3r with wild-type human (MC3RhWT/hWT) and double-mutant (C17A+G241A) human (MC3RhDM/hDM) MC3R, we found that MC3RhDM/hDM have greater weight and fat mass (Figure 2), increased energy intake and feeding efficiency, but reduced length and fat-free mass than MC3RhWT/hWT (Reference 1). MC3RhDM/hDM mice do not have increased adipose tissue inflammatory-cell infiltration or greater expression of inflammatory markers despite their greater fat mass. Serum adiponectin is increased in MC3RhDM/hDM mice and MC3RhDM/hDM human subjects (Figure 2). MC3RhDM/hDM bone- and adipose tissue–derived mesenchymal stem cells (MSCs) differentiate into adipocytes that accumulate more triglyceride than do MC3RhWT/hWT MSCs. MC3RhDM/hDM thus impacts nutrient partitioning to generate increased adipose tissue that appears metabolically healthy. These data confirm the importance of MC3R signaling in human metabolism and suggest a previously unrecognized role for the MC3R in adipose tissue development. Ongoing studies continue to improve our understanding of the phenotype of these mice. We are investigating a novel role for the MC3R in regulating hepatic autophagy, the role of MC3R in stem cell fate, and how variations in Mc3r may alter signaling of several downstream signaling pathways.
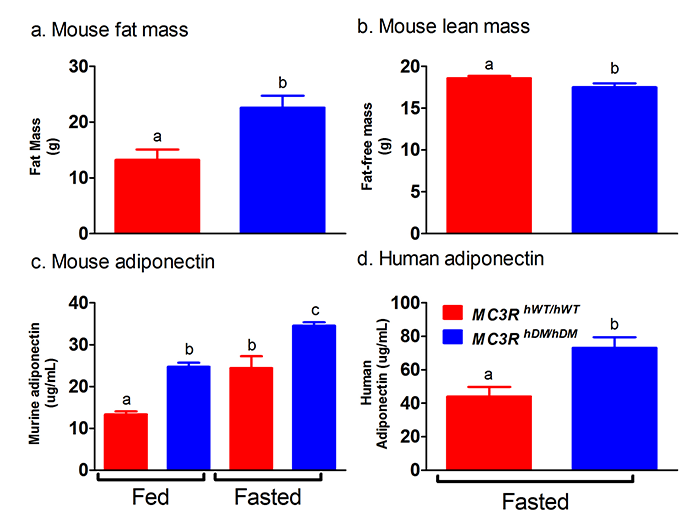
Click image to enlarge.
Figure 2. Studies of a human MC3R variant containing two naturally occurring polymorphisms
The variant is associated with pediatric-onset obesity. We found that mice whose Mc3r was replaced by human versions of the gene were obese when they expressed the double-mutant gene (MC3RhDM/hDM) with greater fat mass (panel a) and lower fat-free mass (panel b), but surprisingly greater adiponectin concentrations (panel c) than mice with the normal human MC3R (MC3RhWT/hWT). Humans with the double-mutant receptor also showed greater adiponectin (panel d).
Physiology, metabolism, and psychology of childhood body weight regulation
Our studies are directed at understanding the physiological, psychological, and metabolic factors that place children at risk for undue weight gain. As part of these studies, we examined how best to measure eating-related psychopathology, insulin sensitivity, changes in body composition, energy intake, and energy expenditure in children, and we studied the short- and long-term stability of the components of metabolic syndrome. We found that leptin is an important predictor of weight gain in children: those with high leptin gain even more weight when followed longitudinally. We also documented that hyperinsulinemia is positively related to energy intake in non-diabetic, obese children, leading to treatment studies to reduce hyperinsulinemia (see below). We also examined the relationship between depressive symptomatology and insulin resistance in children and adolescents, finding strong associations both cross-sectionally and prospectively between depressive symptoms and insulin resistance independent of body weight. The associations suggest mechanisms whereby insulin resistance may contribute to excessive weight gain in children, and they have informed some of our treatment approaches to pediatric obesity (described below).
Our evaluations concentrating on binge-eating behaviors in children suggest that such behaviors also are associated with adiposity in children and abnormalities in metabolism. We found that binge-eating behaviors may predict future weight gain in children at risk for obesity. Children reporting binge-eating behaviors such as loss of control over eating gained, on average, an additional 2.4 kg of weight per year compared with non-binge-eating children. Our data also suggest that children endorsing binge eating consume more energy during meals. Actual intake during buffet meals averaged 400 kcal more in children with binge eating, but despite their greater intake, such children reported shorter-lived satiety than children without binge-eating episodes. The ability to consume large quantities of palatable foods, especially when coupled with lowered subsequent satiety, may play a role in the greater weight gain found in binge-eating children. We demonstrated that, among a cohort of 506 lean and obese youth, youth with loss of control (LOC) over eating had significantly higher serum leptin levels than those without LOC episodes, even after adjusting for adiposity and other relevant covariates. The data also suggest that interventions targeting disordered eating behaviors may be useful in preventing excessive fat gain in children prone to obesity and they have led to trials of preventative strategies related to binge eating. Because binge eating appears to be a heritable trait, we have also initiated studies to investigate possible genetic factors linked to loss of control over eating.
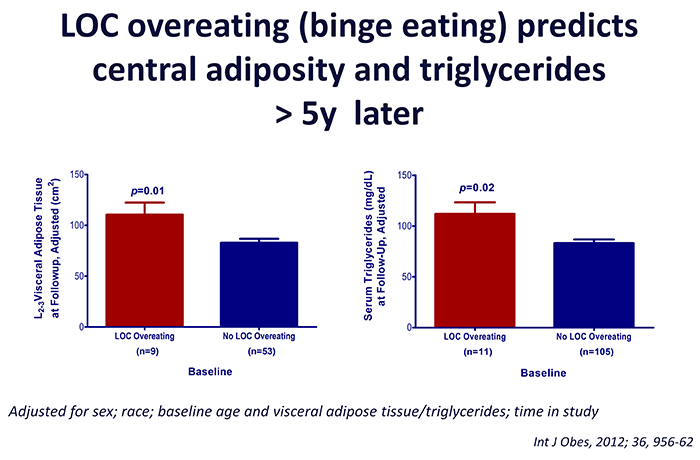
Click image to enlarge.
Figure 3. Loss of control (LOC) eating and metabolic complications in a longitudinal study
On average (±SE), children who engaged in binge eating at baseline had more visceral adipose tissue at L2-3 intervertebral space at follow-up than children who did not engage in binge eating at baseline, adjusting for sex, race, baseline age, baseline visceral adipose tissue at L2-3, and time in study (P = 0.01). On average (±SE), children who engaged in binge eating at baseline had higher follow-up triglycerides than children who did not engage in binge eating at baseline, adjusting for sex, race, baseline age, body mass index (kg/m2), baseline triglycerides, and time in study (P = 0.02).
We study normal-weight children and adolescents, children who are already obese, and the non-obese children of obese parents, in order to determine the factors that are most important for the development of complications of obesity in youth. Body composition, leptin concentration metabolic rate, insulin sensitivity (Reference 2), glucose disposal, energy intake at buffet meals, and genetic factors believed to regulate metabolic rate and body composition are examined. We also study psychological and behavioral factors, such as propensity to engage in binge-eating behavior (Figure 3). Children are being followed longitudinally into adulthood. In two protocols, we study actual food consumption of children during meals, to elucidate differences in the calorie and macronutrient content of meals and the circulating hormones related to hunger and satiety in those who either endorse binge-eating behaviors or report no such behaviors. We found that eating in the absence of physiological hunger is a replicable trait that appears linked to obesity. We also investigated the role of sedentary behaviors, such as television watching, as a factor that alters metabolism. In a randomized, controlled, crossover trial (Figure 4), we found that glucose tolerance was markedly improved in children who engaged in moderate activity for just three minutes every half hour, versus remaining sedentary (Reference 3). We hypothesize that differences in these factors will predict the development of obesity in the populations studied, and may be of great importance in developing rational approaches for the prevention and treatment of obesity in the diverse US population.
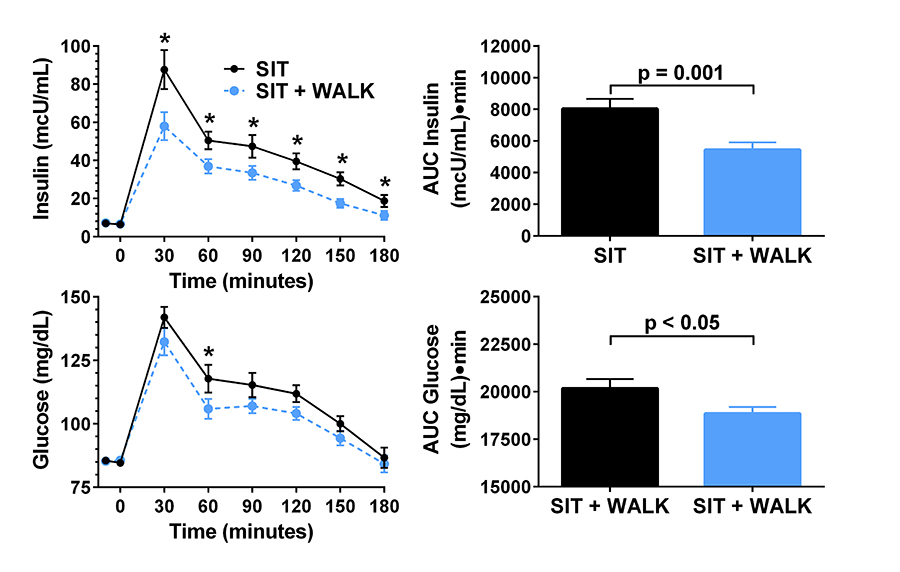
Click image to enlarge.
Figure 4. Effect of short, moderate intensity walking breaks on children’s glucose tolerance
Children who walked for three minutes every 30 minutes (in blue) had lower glucose and insulin concentrations during an oral glucose tolerance test than when they sat uninterrupted for three hours (in black).
Treatment of obesity and the co-morbid conditions associated with obesity
Given the rapid increase in the prevalence of obesity, the development of treatments for obesity in children and adults is urgently needed, yet current pharmacologic approaches are extremely limited, both for children and adults. In several clinical protocols, we examined approaches for the prevention and treatment of excessive body weight. We completed a randomized controlled trial demonstrating that severely obese adolescents can lose weight when enrolled in a comprehensive weight-management program that includes the gastrointestinal lipase inhibitor orlistat as an adjunct to a behavioral modification program. Adolescents treated with orlistat lost significantly more weight, BMI units, and fat mass than those that were not. We concluded that, added to a behavioral program, orlistat significantly improved weight loss over a 6-month interval. However, the drug had little impact on obesity-related co-morbid conditions in obese adolescents.
A second obesity treatment study examined the mechanism by which metformin may affect the body weight of younger children who have hyperinsulinemia and are therefore at risk for later development of type 2 diabetes. Compared with placebo-treated children, those randomized to metformin reduced BMI, BMI-Z score, and body fat mass to significantly greater extents. Serum glucose and HOMA-IR also decreased more in metformin-treated than in placebo-treated children. We recently published studies on the pharmacokinetics of metformin and how polymorphisms in enzymes affecting metformin clearance impact weight change. Mean population apparent clearance (CL/F) was 68.1 L/h, and mean apparent volume of distribution (V/F) was 28.8 L. Body weight was a covariate of CL/F and V/F. Estimated glomerular filtration rate was a significant covariate of CL/F. Carriers of a variant of the polyspecific organic cation transporter gene SLC22A1 had significantly smaller reductions in percentage of total trunk fat after metformin therapy. The median percentage change in trunk fat was –2.20% (–9.00% to 0.900%) and –1.20% (–2.40% to 7.30%) for the normal SLC22A1 subjects and variant carriers, respectively.
A third study examined prevention of weight gain using interpersonal therapy (IPT) versus a control health education program (HE) in adolescents reporting LOC eating behaviors (Reference 4). At three-year follow-up, baseline social-adjustment problems and trait anxiety significantly moderated outcome. Among girls with high self-reported baseline social-adjustment problems or anxiety, IPT was significantly associated with the steepest declines in BMIz compared with HE. For adiposity, girls with high or low anxiety in HE and girls with low anxiety in IPT experienced gains, while girls in IPT with high anxiety stabilized. Parent reports yielded complementary findings. The results have stimulated ongoing research to examine how anxiety may stimulate energy intake. This year, we also published preliminary data from a fourth study examining IPT approaches in younger children, finding good tolerability for such a program. A fifth study examined whether reducing depressive symptoms could ameliorate insulin resistance in adolescents at risk for Type 2 Diabetes (Reference 5). Among girls with greater (moderate) baseline depressive symptoms (N = 78), those in cognitive behavior therapy (CBT) developed lower 2-hr insulin than those in HE. Additional metabolic benefits of CBT were seen for this subgroup in post hoc analyses of post-treatment to one-year change.
We also participated in a multi-site randomized, placebo-controlled trial of beloranib, an inhibitor of methionyl aminopeptidase 2, to treat the hyperphagia of patients with the Prader Willi syndrome (Figure 5). The medication was effective in reducing body weight: compared with placebo, weight change was greater with 1.8 mg (mean difference –8.2%) and 2.4 mg beloranib (–9.5%). Unfortunately, the trial had to be halted due to an imbalance in venous thrombotic events in beloranib-treated participants (two fatal events of pulmonary embolism and two events of deep vein thrombosis) compared with placebo.
This year, we initiated a translational trial studying the effects of modulation of the leptin signaling pathway with the melanocortin agonist setmelanotide in patients with proximal signaling defects such as PCSK1 insufficiency and in patients with Bardet Biedl syndrome. A novel intervention, which should complete enrollment in 2018, is a randomized controlled trial of colchicine to ameliorate the inflammation associated with obesity and thus reduce its complications.
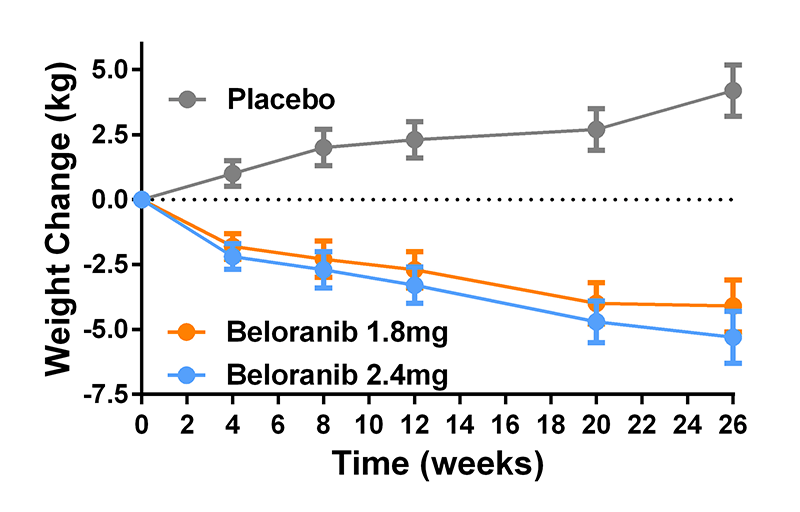
Click image to enlarge.
Figure 5. Effects of placebo and beloranib on body weight in patients with hyperphagia due to the Prader-Willi syndrome
Patients with Prader-Willi syndrome who were treated with the experimental medication beloranib had significantly more weight loss than those treated with placebo, who gained weight during the study.
Additional Funding
- NIH Clinical Center/ORD “Bench to Bedside” Award: Melanocortin agonist to bypass leptin resistance of Bardet-Biedl syndrome. 2017-2018
- NIH Clinical Center/ORWH “Bench to Bedside” Award: Attention Bias Retraining in Adolescents with Loss of Control Eating. 2016-2017
- Office of Disease Prevention, NIH: Grant supplement to support the clinical protocol “Effects of Interrupting Sedentary Behavior on Metabolic and Cognitive Outcomes in Children.” 2016–2017
- Zafgen Inc: “Randomized, Double-Blind, Placebo Controlled, Phase 3 Trial of ZGN-440 (Subcutaneous Beloranib in Suspension, Zafgen, Inc.) in Obese Subjects with Prader-Willi Syndrome to Evaluate Total Body Weight, Food-Related Behavior, and Safety over 6 Months.” Funds an RCT testing a new methionine aminopeptidase inhibitor in patients with Prader-Willi syndrome and obesity. 2014–2016
- Rhythm Pharmaceuticals, Inc: Setmelanotide (RM-493; Rhythm Pharmaceuticals, Inc.) Phase 2 Open-Label Treatment Trials in Patients with Rare Genetic Disorders of Obesity. 2017-2019
Publications
- Lee B, Koo J, Yun Jun J, Gavrilova O, Lee Y, Seo AY, Taylor-Douglas DC, Adler-Wailes DC, Chen F, Gardner R, Koutzoumis D, Sherafat Kazemzadeh R, Roberson RB, Yanovski JA. A mouse model for a partially inactive obesity-associated human MC3R variant. Nat Commun 2016 7:10522.
- Sedaka NM, Olsen CH, Yannai LE, Stutzman WE, Krause AJ, Sherafat-Kazemzadeh R, Condarco TA, Brady SM, Demidowich AP, Reynolds JC, Yanovski SZ, Hubbard VS, Yanovski JA. A longitudinal study of serum insulin and insulin resistance as predictors of weight and body fat gain in African American and Caucasian children. Int J Obes (Lond) 2017 41:61-70.
- Belcher BR, Berrigan D, Papachristopoulou A, Brady SM, Bernstein SB, Brychta RJ, Hattenbach JD, Tigner IL, Courville AB, Drinkard BE, Smith KP, Rosing DR, Wolters PL, Chen KY, Yanovski JA. Effects of interrupting children's sedentary behaviors with activity on metabolic function: a randomized trial. J Clin Endocrinol Metab 2015 100:3735-3743.
- Tanofsky-Kraff M, Shomaker LB, Wilfley DE, Young JF, Sbrocco T, Stephens M, Brady SM, Galescu O, Demidowich A, Olsen CH, Kozlosky M, Reynolds JC, Yanovski JA. Excess weight gain prevention in adolescents: three-year outcome following a randomized controlled trial. J Consult Clin Psychol 2017 85:218-227.
- Shomaker LB, Kelly NR, Radin RM, Cassidy OL, Shank LM, Brady SM, Demidowich AP, Olsen CH, Chen KY, Stice E, Tanofsky-Kraff M, Yanovski JA. Prevention of insulin resistance in adolescents at risk for type 2 diabetes with depressive symptoms: 1-year follow-up of a randomized trial. Depress Anxiety 2017 34:866-876.
Collaborators
- Silva Arslanian, MD, Children’s Hospital of Pittsburgh, Pittsburgh, PA
- Jeffrey Baron, MD, Section on Growth and Development, NICHD, Bethesda, MD
- Andrew Butler, PhD, The Scripps Research Institute, La Jolla, CA
- Kong Chen, PhD, Clinical Endocrinology Branch, NIDDK, Bethesda, MD
- Anthony Comuzzie, PhD, Southwest National Primate Research Center, San Antonio, Texas
- I. Sadaf Farooqi, MD, Cambridge Institute for Medical Research, Cambridge, United Kingdom
- Oksana Gavrilova, PhD, Mouse Metabolism Core Laboratory, NIDDK, Bethesda, MD
- Joan C. Han, MD, Le Bonheur Children’s Hospital, Memphis, TN
- Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Baton Rouge, LA
- Michael Jensen, MD, Mayo Clinic, Rochester, MN
- Rudolph L. Leibel, MD, Columbia University College of Physicians and Surgeons, New York, NY
- Sergey Leikin, PhD, Section on Physical Biochemistry, NICHD, Bethesda, MD
- David S. Ludwig, MD, PhD, Children’s Hospital, Boston, Boston MA
- Cara Olsen, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Daniel Pine, MD, Section on Development and Affective Neuroscience, NIMH, Bethesda, MD
- Douglas Rosing, MD, Cardiac Consultation Service, NHLBI, Bethesda, MD
- Peter J. Schmidt, MD, Section on Behavioral Endocrinology, NIMH, Bethesda, MD
- Lauren B. Shomaker, PhD, University of Colorado, Boulder, CO
- Eric Stice, PhD, Oregon Research Institute, Eugene, OR
- Marian Tanofsky-Kraff, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- B. Timothy Walsh, PhD, Columbia University College of Physicians and Surgeons, New York, NY
- Denise E. Wilfley, PhD, Washington University School of Medicine, St. Louis, MO
- Joshua Zimmerberg, MD, PhD, Section on Integrative Biophysics, NICHD, Bethesda, MD
Contact
For more information, email yanovskj@mail.nih.gov or visit http://sgo.nichd.nih.gov.


