Hippocampal Interneurons and Their Role in the Control of Network Excitability

- Chris J. McBain, PhD, Head, Section on Cellular and Synaptic Physiology
- Ramesh Chittajulla, PhD, Staff Scientist
- Kenneth Pelkey, PhD, Staff Scientist
- Gulcan Akgul, PhD, Postdoctoral Fellow
- James D'Amour, PhD, Postdoctoral Fellow
- Vivek Madahavan, PhD, Postdoctoral Fellow
- Jason Wester, PhD, Postdoctoral Fellow
- Steven Hunt, Biologist
- Xiaoqing Yuan, MSc, Biologist
- Daniela Calvigioni, BSc, Graduate Student
- Tyler Ekins, BSc, Predoctoral Fellow
Cortical and hippocampal local-circuit GABAergic inhibitory interneurons are ‘tailor-made’ to control Na+- and Ca2+-dependent action potential generation, regulate synaptic transmission and plasticity, and pace large-scale synchronous oscillatory activity. The axons of this diverse cell population make local, usually short-range projections (some subpopulations project their axons over considerable distances) and release the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) onto a variety of targets. A mounting appreciation of the roles played by interneurons in several mental health conditions such as epilepsy, stroke, Alzheimer’s disease, and schizophrenia have placed this important cell type center stage in cortical circuit research. Our main objective is to understand the developmental programs that regulate their integration into cortical circuits and how both ionic and synaptic mechanisms regulate the activity of cortical neurons at the level of small, well defined networks. To this end, we use a variety of electrophysiological, immunohistochemical, molecular, and genetic approaches in both wild-type and transgenic animals. Over the past few years, we have continued our study on the differential mechanisms of glutamatergic and GABAergic synaptic transmission and plasticity within the hippocampal formation and the modulation of voltage- and ligand-gated channels expressed in inhibitory neurons. We also incorporate genetic approaches to unravel the embryogenesis and development of hippocampal interneurons and the circuits in which they are embedded. We are particularly interested in discovering the rules that dictate coordinated protein expression in nascent interneuron subpopulations as they migrate and integrate into the developing cortical circuit.
Afferent specific role of NMDA receptors for the circuit integration of hippocampal neurogliaform cells.
Appropriate integration of GABAergic interneurons into nascent cortical circuits is critical for ensuring normal information processing within the brain. Network and cognitive deficits associated with neurological disorders, such as schizophrenia, that result from NMDA receptor (NMDAR) hypofunction have been mainly attributed to dysfunction of parvalbumin-expressing interneurons that paradoxically express low levels of synaptic NMDARs. We revealed that, throughout postnatal development, thalamic and entorhinal cortical inputs onto hippocampal neurogliaform cells are characterized by a large NMDAR–mediated component. This NMDAR signaling is prerequisite for developmental programs ultimately responsible for the appropriate long-range glutamate receptor (AMPAR)–mediated recruitment of neurogliaform cells. In contrast, AMPAR–mediated input at local Schaffer-collateral synapses on neurogliaform cells remains normal following NMDAR ablation. These afferent specific deficits potentially impact neurogliaform cell–mediated inhibition within the hippocampus, and our findings reveal circuit loci implicating this relatively understudied interneuron subtype in the etiology of neuro-developmental disorders characterized by NMDAR hypofunction. Proper brain function depends on the correct assembly of excitatory and inhibitory neurons into neural circuits. We showed that, during early postnatal development in mice, NMDAR signaling via activity of long-range synaptic inputs onto neurogliaform cells is required for the cells’ appropriate integration into the hippocampal circuitry.
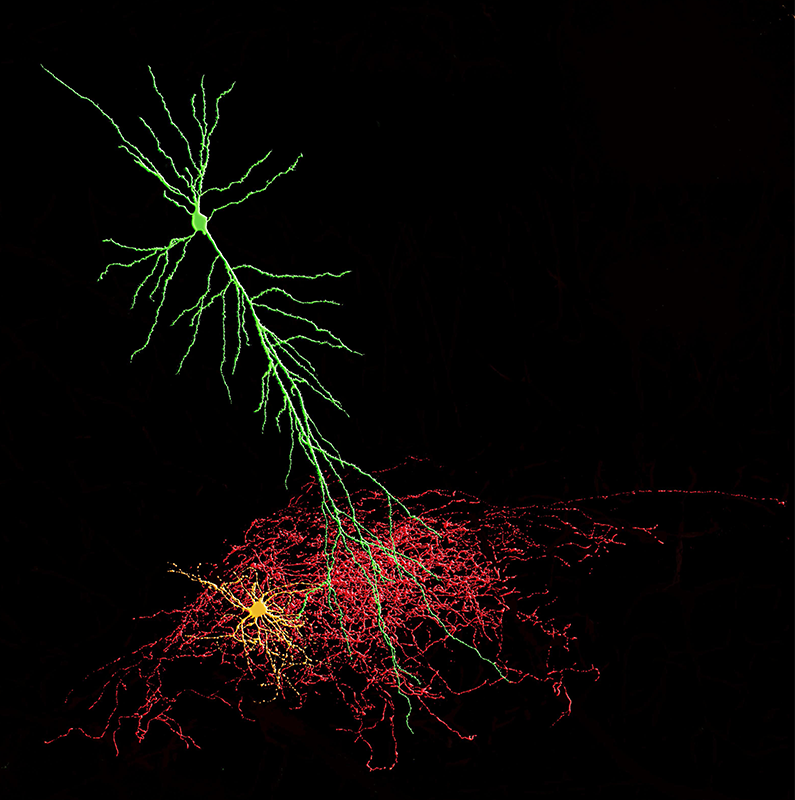
Click image to enlarge.
Neurogliaform-pyramidal cell connection
The axon of a neurogliaform inhibitory interneuron (cell body and dendrites shown in yellow, axon in red) forms a dense spider-web plexus (the cell was originally termed the arachniform cell by the Spanish classic anatomist Ramon y Cajal over a century ago) that wraps around the distal dendrites of a glutamatergic pyramidal cell (shown in green) in the mammalian hippocampus. Cells were recorded using patch-clamp electrophysiological techniques, filled with neurobiotin and recovered using Imaris software.
Neto auxiliary subunits regulate interneuron somatodendritic and presynaptic kainate receptors to control network inhibition.
Although Netos are considered auxiliary subunits critical for kainate receptor (KAR) function, direct evidence for their regulation of native KARs is limited. Because Neto KAR regulation is specific for the KAR–subunit GluK/Neto isoform, such regulation must be determined in cell type–specific contexts. We demonstrate Neto1/2 expression in somatostatin (SOM)-, cholecystokinin/cannabinoid receptor 1 (CCK/CB1)–, and parvalbumin (PV)-containing interneurons. KAR–mediated excitation of these interneurons is contingent upon Neto1 because kainate yields comparable effects in Neto2 knockouts and wild-types but fails to excite interneurons or recruit inhibition in Neto1 knockouts. In contrast, presynaptic KARs in CCK/CB1 interneurons are dually regulated by both Neto1 and Neto2. Neto association promotes tonic presynaptic KAR activation, dampening CCK/CB1 interneuron output, and the loss of this brake in Neto mutants profoundly increases CCK/CB1 interneuron–mediated inhibition. Our results confirm that Neto1 regulates endogenous somatodendritic KARs in diverse interneurons and demonstrate Neto regulation of presynaptic KARs in mature inhibitory presynaptic terminals.
Additional Funding
- Jason Wester was funded by an NINDS Intramural National Research Service Award (NRSA)
- James D’Amour was funded by an NIGMS PRAT Fellowship
Publications
- Wester J, McBain CJ. Inhibitory interneurons differentially contribute to spontaneous network activity in the developing hippocampus dependent on their embryonic lineage. J Neurosci 2016 36:2646-2662.
- Vargish GA, Pelkey KA, Yuan X, Chittajallu R, Collins D, Fang C, McBain CJ. Persistent inhibitory circuit defects and disrupted social behavior following in utero exogenous cannabinoid exposure. Mol Psychiatry 2016 21(1):56-67.
- Perszyk RE, DiRaddo JO, Strong KL, Low C-M, Ogden KK, Khatri A, Vargish GA, Pelkey KA, Tricoire L, Liotta DC, Smith Y, McBain CJ, Traynelis SF. GluN2D-containing NMDA receptors mediate synaptic transmission in hippocampal interneurons. Mol Pharmacol 2016 90:689-702.
- Calvigioni D, Máté Z, Fuzik J, Girach F, Zhang M-D, Varro A, Beiersdorf J, Schwindling C, Yanagawa Y, Dockray G, McBain CJ, Hokfelt T, Szabó G, Keimpema E, Harkany T. Functional differentiation of cholecystokinin-containing interneurons destined for the cerebral cortex. Cereb Cortex 2017 27:2453-2468.
- Xiao M-F, Xu D, Craig MT, Pelkey KA, Chien C-C, Shi Y, Zhang J, Resnick S, Pletnikova O, Salmon D, Brewer J, Edland S, Wegiel J, Tycko B, Savonenko A, Reeves RH, Troncoso JC, McBain CJ, Galasko D, Worley PF. NPTX2 and cognitive dysfunction in Alzheimer’s disease. eLife 2017 6:e23798.
- Chittajallu R, Wester JC, Craig MT, Barksdale E, Yuan X, Akgul G, Fang C, Collins D, Hunt S, Pelkey KA, McBain CJ. Afferent specific role of NMDA receptors for the circuit integration of hippocampal neurogliaform cells. Nat Commun 2017 8(1):152.
- Wyeth MS, Pelkey KA, Yuan X, Vargish G, Johnston A, Lipina TV, Kanisek M, Hunt S, Fang C, Abebe D, Mahadevan V, Fisahn A, Roder JC, Woodin MA, Salter MW, McInnes RR, Chittajallu R, McBain CJ. Neto auxiliary subunits regulate interneuron somatodendritic and presynaptic kainate receptors to control network inhibition. Cell Rep 2017 20(9):2156-2168.
Collaborators
- Tibor Harkany, PhD, Karolinska Institute, Stockholm, Sweden
- Roderick McInnes, PhD, Lady Davis Research Institute, McGill University, Montreal, Canada
- Michael Salter, PhD, Centre for the Study of Pain, Hospital for Sick Children, Toronto, Canada
- Stephen J. Traynelis, PhD, Emory University, Atlanta, GA
- Melanie Woodin, PhD, University Toronto, Toronto Canada
- Paul Worley, PhD, The Johns Hopkins University, Baltimore, MD
Contact
For more information, email mcbainc@mail.nih.gov or visit https://neuroscience.nih.gov/Faculty/Profile/chris-mcbain.aspx.


