Quantitative Biophotonics for Tissue Characterization and Function

- Amir H. Gandjbakhche, PhD, Head, Section on Analytical and Functional Biophotonics
- Fatima A. Chowdhry, MD, Research Fellow
- Afrouz Anderson, PhD, Postdoctoral Fellow
- Elizabeth Smith, PhD, Postdoctoral Fellow
- Nader Shahni Karamzadeh, PhD, Data Scientist (Contractor)
- Ali Afshari, MS, Predoctoral Student
- Hadis Dashtestani, MS, Predoctoral Student
- Riley Kermanian, BS, Postbaccalaureate Fellow
- Kian Parsa, BS, Postbaccalaureate Fellow
- Rachel Zaragoza, BS, Postbaccalaureate Fellow
- Maryam Ghasemi Ghonchehnazi, MSc, Special Volunteer
- Elahe Shafiei Haghshenas, MSc, Special Volunteer
- Franck Amyot, PhD, Guest Researcher
- Siamak Aram, PhD, Guest Researcher
- Yasaman Ardeshirpour, PhD, Guest Researcher
- Bahar Dasgeb, MD, Guest Researcher
- Jana Kainerstorfer, PhD, Guest Researcher
Our general goal is to devise quantitative methodologies and associated instrumentation to bring technologies from the bench to bedside. We select our projects in the framework of the mission of the NICHD, focusing on childhood and adolescent disorders, as well as abnormal developments of cells such as occur in cancer. We operate in the so-called 4B research mode: (1) at the Blackboard—modeling the methodologies; (2) at the Bench—designing the prototypes to be brought to the Bedside; (3) at the Bedside—piloting clinical protocols; and (4) Back to the Blackboard—improving the systems/methodologies.
Structural and functional brain imaging
Functional near-infrared spectroscopy (fNIRS) is an emerging, non-invasive imaging technique to assess the brain function. Because the technique is non-invasive and portable it is applicable to studies of children and toddlers, especially those with neuro-developmental disorders. fNIRS measurements are based on the local changes in cerebral hemodynamic levels (oxy-hemoglobin and deoxy-hemoglobin) associated with brain activity and function similar to BOLD fMRI. Near-infrared (NIR) light can penetrate enough to probe the cortical regions up to 1–3 cm deep. fNIRS is portable, low cost, and tolerant of patient movement. We address the changes in NIRS signal in relation to underlying physiological processes in the brain, such as cerebral autoregulation, and by using the technique to study cortical hemodynamics based on individual differences and development. A novel method of data processing to enrich informational content of measured characteristics from fNIRS is therefore crucial for further studies of brain function and development.
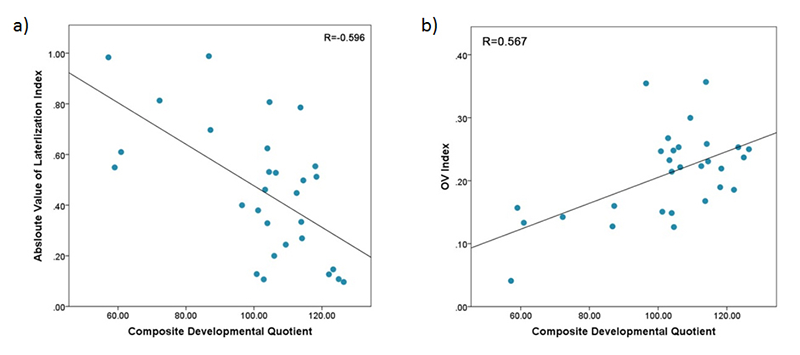
In one of our pilot studies, we evaluated the utility of fNIRS for measuring cerebral hemodynamics in the prefrontal cortex (PFC) in toddlers between 18–36 months of age. We also analyzed the correlation between fNIRS measures and the Composite Developmental Quotient (Composite-DQ) in toddlers with typical development and in those at risk (AR) for developmental delay, during a resting period. The analysis includes assessment of laterality index (percentage difference between brain activation in left and right prefrontal cortex), and the oxygenation variability (OV) index, which quantifies the variability in oxygen saturation at frequencies attributed to cerebral autoregulation for each child. Cerebral autoregulation maintains the blood flow over the range of arterial blood pressure and is vital for brain function and development. The Composite-DQ was calculated based on the average of non-verbal and verbal developmental quotients. We found that toddlers with a lower Composite-DQ (AR) exhibited significantly more rightward activation. Moreover, toddlers with a lower Composite-DQ showed a greater discrepancy between left and right hemisphere activity, with a significant negative correlation between Composite-DQ and the absolute value of the laterality index (Figure 1a). In addition, toddlers with lower developmental scores showed a significantly lower OV index with those with higher scores (Figure 1b). While this study suggests that some features of prefrontal hemodynamics may vary in toddlers at risk for developmental delays due to early language delay, more research in this age group is required to clarify the specificity of these differences. These preliminary findings show the feasibility of using fNIRS in typical toddlers and those with delayed development and, in doing so, support future studies in larger samples.
Click image to enlarge.
Figure 1.
a) Correlation between Absolute values of Laterlization Index and Composite-Developmental Quotient (DQ) indicating increased differences between left and right prefrontal cortex (PFC) activation in subjects with lower Composite-DQ.
b) Correlation between oxygenation variability (OV) index values combined across Left and Right prefrontal cortex and Composite-DQ across all toddlers exhibiting trend of higher OV index in toddlers with higher Composite-DQ.
We further examined the effects of individual differences on the underlying neural process of working memory (WM) tasks. Using fNIRS, we administered a visual and auditory n-back task to examine activation in the PFC, while considering the influences of age, task performance, and learning strategy (VARK score) in adults. Results showed age-related activation increase in the left and right medial PFC. Aside from age differences, high performance (HP) subjects (accuracy greater than 90%) showed lower activation than normal performance subjects. After accounting for learning style, we found a correlation between visual and aural VARK score and the level of activation in the PFC. Subjects with higher visual VARK scores displayed lower activation during auditory stimuli in the left dorsolateral PFC (DLPFC), while those with higher visual scores exhibited higher activation during auditory stimuli in bilateral DLPFC. Auditory HP subjects had higher visual VARK scores, indicating an effect of learning style on task performance and activation. The results of this study show that learning style and task performance can influence PFC activation, with applications toward and implications for neurological learning style and populations with deficits in auditory or visual processing.
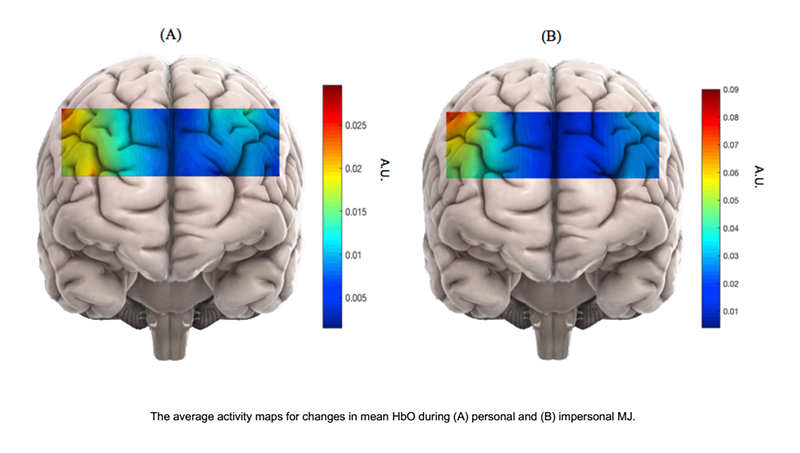
Using fNIRS, we also study antisocial personality disorder (ASPD), which is characterized by a violation of the rights of others and lack of conformity to social norms. ASPD is prevalent in incarcerated populations and often goes undiagnosed. This poses a high cost on society, which indicates the critical need for early detection of ASPD to implement treatments. While ASPD is traditionally diagnosed through psychiatric evaluation in accordance with the symptoms outlined in DSMV, ASPD patients can be extremely manipulative, resulting in controversial diagnoses by subjective measures. However, understanding the neural basis behind ASPD can greatly enhance traditional diagnostic methods. Scientists have also found correlations between psychopathic personality traits and responses to moral judgment (MJ) tasks. Our study would be the first neuroimaging study to implement the MJ task with personality assessments of psychopathic traits in a cost-effective manner and a patient-friendly environment. We will utilize fNIRS to measure brain activation by monitoring changes of oxygenated hemoglobin in the brain similar to fMRI-BOLD. The task used in our study of MJ is based on a series of questions that examine personal versus impersonal dilemmas, defined as emotionally salient scenarios versus more distant ones. We hypothesized that the brain exhibits distinguishable hemodynamic patterns for each category. We will also investigate the correlation between these patterns and psychopathic traits. Using the hemodynamic responses of typical subjects, we will analyze the fNIRS data using a non-linear classification method called cubic SVM. We specifically chose SVM because it determines the separating hyperplane (high dimensional analog to the plane separating the two groups) only from signals located close to the interface between personal and impersonal hemodynamic responses. This should confirm our hypothesis of distinguishable hemodynamic patterns by category and suggests that it is possible to classify degrees of psychopathy based on neural activity. Consequently, we offer a novel approach to provide functional biomarker for ASPD using fNIRS, combined with advanced machine-learning techniques. In the future, we plan to apply our method to incarcerated populations to assess the degree of ASPD (Figure 2).
Multispectral imaging
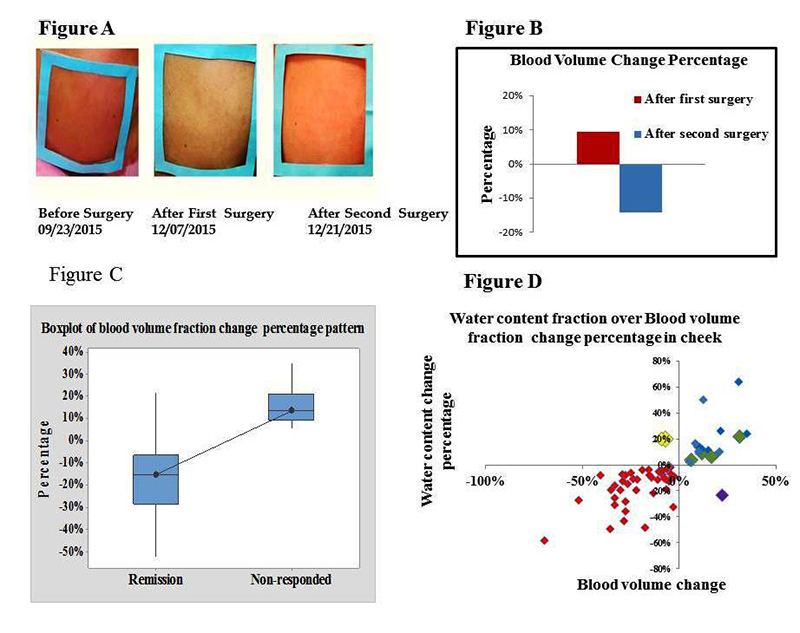
Facial plethora is one of the earliest described clinical features of Cushing's syndrome (CS). In collaboration with the Stratakis group, we continued to quantify changes of facial plethora in CS as an early assessment of cure. Cushing patients are recruited for optical imaging, before and after surgery, with follow-up sessions at six months and two years after surgery. We were able to reconstruct biological parameters of 73 subjects. Non-invasive, multi-spectral, near-infrared imaging was performed on the right cheek of a patient before and two to 14 days after surgery. Patients were defined as cured by post-operative measurements of plasma cortisol less than 3 mcg/dl and/or adrenocortical insufficiency, for which they received replacement. Clinical data obtained from 64 patients indicate that a reduction in facial plethora after surgery, as evidenced by a lowered blood volume fraction, is well correlated with cure of CS (Reference 6). Our recent findings show that, as a new biomarker, water content fraction could also quantify facial plethora. Using this information, we were able to distinguish between cured and non-cured CS patients within a short time after surgical treatment. The findings for both blood volume and water content fractions correlated significantly, but it appears that blood volume is better associated with clinical evaluations; the number of false-negatives in water content analysis is slightly higher. We ran our methodology and analytic algorithm for first and second post-surgery follow up. We processed data for 22 imaged patients from their first follow up (3 to 6 months after surgery) and ten patients in their second follow up (6 months after the first follow up). So far, all have been identified clinically as in remission by their last visit. The results confirmed our hypothesis that the blood volume fraction in the region of interest for patients who were assessed as cured, based on significant drop in their cortisol level after the surgery, was lower than before surgical treatment.
Click image to enlarge.
Figure 3.
A) Facial plethora in a patient with Cushing's disease (CD) before trans-sphenoidal surgery (TSS), after the first TSS (non-cured), and after the second TSS (cured).
B) The blood volume fraction percentage change of the patient's right cheek associated with Figure A at different imaging sessions underscores the advantage of the method over visual inspection.
C) Boxplot of medium percentage change indicating change in blood volume after surgery, compared with before surgery, in each of the remission and non-responding groups of CD patients. The distribution shows median confidence interval box and interquartile range.
D) Correlation of percentage change in blood volume and water content fractions of right cheek side of 57 CD patients who received surgical treatment.
We are pursuing Kaposi Sarcoma (KS) studies in three ongoing NCI clinical trials. After processing more patients under a new classification and with the results of supporting the capability of our novel technology as a robust device, the goal is to further evaluate diffuse multispectral imaging in a relatively large sample as a potential supplement to existing response assessment in KS. In our preliminary experiments, multi-spectral images of KS skin lesions were made over the course of treatment. Blood volume and oxygenation concentration maps were obtained through Principal Component Analysis (PCA). We compared corresponding images with conventional clinical and pathological assessment. In agreement with our hypothesis that successful treatment would reduce the blood volume in the lesions, the normalized standard deviation of blood volume decreased in each of the eight patients whose lesion responded to treatment, while it increased in two patients whose lesion did not respond to therapy. These initial results confirm that concentrations of oxygenated hemoglobin in the tumor can be used as a quantitative marker of tumor response to therapy.
Fluorescence methods in pre-clinical studies of HER2–positive breast cancer and of basal cell carcinoma expressing BerEP4
HER2–specific fluorescently labeled probes
We studied the potential of in vivo fluorescence lifetime imaging to monitor the efficacy of treatment, in particular the feasibility of fluorescence lifetime imaging to monitor in vivo expression of the HER2 receptor in a breast carcinoma (mouse model) during the course of treatment. We observed a considerable difference between the fluorescence lifetime of HER2–specific optical probes at the tumor and a contra-lateral site before and seven days after the last treatment with 17-DMAG (a heat shock protein 90 [HSP90] inhibitor), when the tumor regrew to almost its pretreatment volume. However, soon after the therapy (12 hours), when the effect of the drug on HER2 degradation is maximal, the difference was significantly smaller. Immediately after treatment, the fraction of bound to total fluorophores inside the tumor changed considerably, resulting in a noticeable increase in the average fluorescence lifetime. Subsequent tumor and HER2 expression recovery a week later caused gradual restoration of the original level of the binding ratio of HER2–targeting probe in the tumor and a corresponding return to pre-treatment values of the fluorescence lifetime. The results reveal that fluorescence lifetime imaging, based on evaluating the fraction of the bound and unbound fluorophores inside the tumor, can be used as an alternative in vivo imaging approach to characterize tumors, separate high from low HER2–expression tumors, and monitor the efficacy of targeted therapies.
Molecular biomarkers for basal cell carcinoma (BCC) for use in its diagnosis, treatment, and follow up
We conducted a pilot study to determine the affinity and selectivity of the BerEp4 antibody conjugated with a fluorescence probe and to assess its possible use in designing theranostic probes for BCC. Based on initial cell culture results, BerEP4 appears to be a promising biomarker for molecular imaging of BCC and can be used in conjunction with in vivo near-infrared fluorescence imaging. To prepare BerEP4 for eventual theranostic use, we examined the feasibility of a combined macro-/micro-optical approach to imaging BCC with various histologies. During an in vitro phase, we showed specificity and selectivity of the BerEP4 antibody to target EpCAM on live cells. EpCAM is an ideal biomarker to target BCC because it is expressed in more than 95% of human BCC. In the subsequent in vivo phase, using xenograft mouse models, we reproduced persisting in vivo specificity and selectivity of the BerEP4 antibody to detect EpCAM–expressing xenograft tumors, when injected systemically via tail veins. Moving forward, we plan to: (1) modify our antibody to a smaller molecule—a stable aptamer or single-strand antibody—to facilitate topical delivery skin cancer; (2) repeat the in vivo experiment to show reproducible results with a modified and smaller probe; this phase would encompass initial systemic injection of the probe followed by experiments to test how best to achieve topical delivery, including micro-injection; (3) once in vivo non-invasive targeting of BCC is achieved via topical introduction, to further engineer our diagnostic probe to be photo-sensitized to achieve photodynamic treatment at the time of diagnosis.
Real-time oximetry of the anterior placenta using near-infrared spectroscopy
Monitoring placental oxygenation is critical to ensure a healthy pregnancy outcome. Problems with utero-placental perfusion have been associated with preeclampsia and intrauterine growth restriction and can lead to fetal hypoxia and cerebral palsy. Currently, there are no patient-friendly devices to measure the oxygenation of the anterior placenta. Therefore, it would be important to have a quantitative understanding of placental oxygenation to detect any abnormality compared to normal placental function. Near-infrared spectroscopy (NIRS) is a technique that can address these challenges with its wearable, wireless capability, which is convenient for dynamic monitoring. We intend to first find the baseline for the normal vs. abnormal pregnancies and to standardize the oxygenation data across pregnancies and then to correlate the oxygenation data with the pregnancy outcomes. To achieve these aims, we designed a non-invasive and wearable device with wireless capability to allow continuous measurement of the oxygenation of the anterior placenta in a subject-friendly environment. This compact system can be positioned at different abdominal locations for localized measurement of oxygenation (Figure 4). The NIRS device uses the light in near-infrared region at two wavelengths (760 and 850 nm). The light enters the tissue at the location of source and the back-scattered light, which is sensitive to changes in oxy- and deoxy-hemoglobin, is detected at the detector sites. The device consists of two detectors and three sources, which permits probing different tissue depth. This would provide us with information regarding different tissue types and to distinguish between maternal and placental tissue.

Click image to enlarge.
Figure 4.
Wearable NIRS device and its schematics for placental oxygenation measurement. The device can be easily applied to different abdominal regions, and near-infrared light from the device will penetrate to different tissue depths.
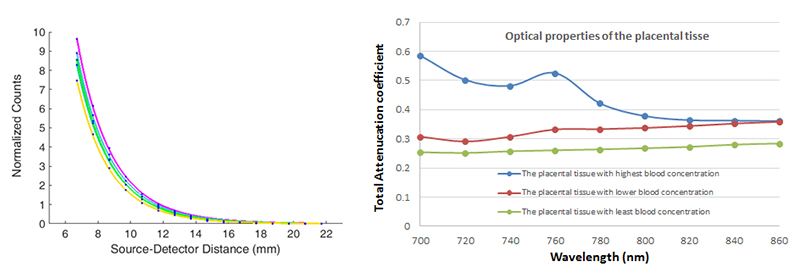
We are also investigating the efficiency of the device to accurately distinguish between the oxygenation of the maternal and the placental tissue. Given that the light passes through several tissue compartments, we developed the multi-layer model that includes the effect of tissue types based on optical properties of skin (melanin concentration), fat (BMI), uterus, and placental tissue. This model is essential, given that calculation of the oxygenation requires prior knowledge of the optical properties of the given tissues, such as scattering and absorption. To determine the optical properties of the placenta, we use an in-house photodiode array unit combined with the laser system. The attenuation coefficient (as a function of scattering and absorption coefficient) for several near-infrared wavelengths is calculated based on the reflection curve (photon count as a function of source-detector distance) from the placental tissues with and without blood (Figure 5). Using Monte Carlo simulation along with our multi-layer model, we developed a system that includes parameters such as skin color, BMI, and uterus thickness into the calculation of oxygenation index.
In collaboration with Roberto Romero, Sonia Hassan, and Shad Deering, we plan to test our device through pilot studies to recruit pregnant subjects. The aim is to find a baseline of placental oxygenation in normal pregnancy. This involves refining our data analysis software by incorporating anatomical localization and standardizing it across pregnancy. We expect to thereby provide earlier detection of pregnancy complications, which can improve both maternal and fetal health.
Click image to enlarge.
Figure 5.
Example of fitted curves based on photon counts as function of source-detector distance for several wavelengths that yield the values of total attenuation coefficients, which contain information about scattering and absorption of the tissue. Right: Total attenuation coefficient as a function of wavelength for the placental tissues with various blood content measured by the system.
Additional Funding
- Bench to Bedside Award 345 (2016): “Mirror neuron network dysfunction as an early biomarker of neurodevelopment” (Ongoing)
- Human Placenta Project-NICHD (2016) (Ongoing)
Publications
- Chue-Sang J, Bai Y, Stoff S, Gonzalez M, Holness N, Gomes J, Jung R, Gandjbakhche A, Chernomordik VV, Ramella-Roman JC. Use of Mueller matrix polarimetry and optical coherence tomography in the characterization of cervical collagen anisotropy. J Biomed Opt 2017 22:1-9.
- Smith E, Anderson A, Thurm A, Shaw P, Maeda M, Chowdhry F, Chernomordik V, Gandjbakhche A. Prefrontal activation during executive tasks emerges over early childhood: evidence from Functional Near Infrared Spectroscopy. Dev Neuropsychol 2017 42:253-264.
- Anderson AA, Smith E, Chowdhry FA, Thurm A, Condy E, Swineford L, Manwaring SS, Amyot F, Matthews D, Gandjbakhche AH. Prefrontal hemodynamics in toddlers at rest: a pilot study of developmental variability. Front Neurosci 2017 11:300-310.
- Karamzadeh N, Amyot F, Kenney K, Anderson A, Chowdhry F, Dashtestani H, Wassermann EM, Chernomordik V, Boccara C, Wegman E, Diaz-Arrastia R, Gandjbakhche AH. A machine learning approach to identify functional biomarkers in human prefrontal cortex for individuals with traumatic brain injury using functional near-infrared spectroscopy. Brain Behav 2016 6(11):e00541.
- Chernomordik V, Amyot F, Kenney K, Wassermann E, Diaz-Arrastia R, Gandjbakhche A. Abnormality of low frequency cerebral hemodynamics oscillations in TBI population. Brain Res 2016 1639:194-199.
- Afshari A, Ardeshirpour Y, Lodish MB, Gourgari E, Sinaii N, Keil M, Belyavskaya E, Lyssikatos C, Chowdhry FA, Chernomordik V, Anderson AA, Mazzuchi TA, Gandjbakhche A, Stratakis CA. Facial plethora: modern technology for quantifying an ancient clinical sign and its use in Cushing syndrome. J Clin Endocrinol Metab 2015 100(10):3928-3933.
Collaborators
- Claude Boccara, PhD, École Supérieure de Physique et de Chimie Industrielles, Paris, France
- Shad Deering, MD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Stavros Demos, PhD, Lawrence Livermore National Laboratory, Livermore, CA
- Ramon Diaz-Arrastia, MD, PhD, Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD
- Israel Gannot, PhD, Tel Aviv University, Tel Aviv, Israel, and Johns Hopkins University, Baltimore, MD
- Sonia S. Hassan, MD, Wayne State University School of Medicine, Detroit, MI
- Jay Knutson, PhD, Laboratory of Molecular Biophysics, NHLBI, Bethesda, MD
- Maya Lodish, MD, Pediatric Endocrinology Inter-InstituteTraining Program, NICHD, Bethesda, MD
- Tom Pohida, MS, Division of Computational Bioscience, Center for Information Technology, NIH, Bethesda, MD
- Randall Pursley, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Jessica C. Ramella-Roman, PhD, Florida International University, Miami, FL
- Roberto Romero-Galue, MD, Perinatology Research Branch, NICHD, Detroit, MI
- Dan Sackett, PhD, Division of Basic and Translational Biophysics, NICHD, Bethesda, MD
- Babak Shadgan, MD, MSc, PhD, University of British Columbia, Vancouver, Canada
- Constantine Stratakis, MD, D(med)Sci, Section on Endocrinology and Genetics, NICHD, Bethesda, MD
- Audrey Thurm, PhD, Pediatrics & Developmental Neuropsychiatry Branch, NIMH, Bethesda, MD
- Eric Wassermann, MD, Cognitive Neuroscience Section, NINDS, Bethesda, MD
- Robert Yarchoan, MD, HIV and AIDS Malignancy Branch, NCI, Bethesda, MD
Contact
For more information, email amir@helix.nih.gov or visit http://safb.nichd.nih.gov.