Fusion mediated by HIV-1 Env and by HAP2 gamete protein fusogens

- Leonid V. Chernomordik, PhD, Head, Section on Membrane Biology
- Eugenia Leikina, DVM, Senior Research Assistant
- Kamram Melikov, PhD, Staff Scientist
- Elena Zaitseva, PhD, Staff Scientist
- Santosh K. Verma, PhD, Visiting Fellow
- Berna Uygur, PhD, Postdoctoral Intramural Research Training Award Fellow
- Kepler Mears, BS, Postbaccalaureate Fellow
Disparate membrane remodeling reactions are tightly controlled by protein machinery but are also dependent on the lipid composition of the membranes. Whereas each kind of protein has an individual personality, membrane lipid bilayers have rather general properties manifested by their resistance to disruption and bending. Our long-term goal is to understand how proteins break and reseal membrane lipid bilayers in important cell biology processes, such as membrane fusion and the crossing of cell membranes by water-soluble drugs on their way to intracellular targets. We expect that the analysis of the molecular mechanisms of various membrane rearrangements will clarify the generality of emerging mechanistic insights. Better understanding of these mechanisms will bring about new ways to control them and lead to new strategies for treating diseases involving cell invasion by enveloped viruses, intracellular trafficking, and intercellular fusion. In addition to our on-going work on the fusion stages in the formation of multi-nucleated myotubes and osteoclasts, where even the identities of proteins involved remain to be clarified, in two recent studies we focused on the HIV-1–mediated fusion stage and on characterization of the gamete fusogen HAP2.
The fusion stage of HIV-1 entry depends on virus-induced cell-surface exposure of phosphatidylserine.
Human Immunodeficiency virus 1 (HIV-1), the causative agent of AIDS, delivers its RNA into cells by fusing the viral envelope with the cell membrane. The fusion process is mediated by the viral envelope glycoprotein Env, a trimer of heterodimers consisting of gp120 and gp41 subunits. Fusion is initiated by gp120 interactions with the CD4 receptor and one of the two G protein–coupled receptors (GPCR) coreceptors, CCR5 and CXCR4 at the surface of target cells. Several studies, particularly those on resting primary CD4 T cells, have suggested that efficient Env–mediated fusion and infection also depends on intracellular signaling. Specifically, Ca2+ signaling is triggered by engagement of the coreceptors with gp120. However, the role of signaling in HIV-1 fusion and infection remains controversial and appears to be dependent on cell type and activation status.
In collaboration with Leonid Margolis and Gregory Melikian, we recently reported that HIV-1 binding to its receptors induces non-apoptotic exposure of phosphatidylserine (PS) at the surface of the target cell and that externalized PS strongly promotes Env–mediated membrane fusion and HIV-1 infection (Figure 1 and Reference 2). Specific interactions between the gp120 subunit of Env and coreceptors triggered Ca2+ signaling–dependent transmembrane protein 16 (TMEM16F)–mediated PS externalization. Blocking externalized PS with PS–binding proteins or suppressing TMEM16F function inhibited Env–mediated fusion at a stage that follows the formation of pre-fusion Env–CD4–coreceptor complexes and precedes gp41 restructuring, which brings about hemifusion and fusion. Exogenous PS (exoPS) added to the outer leaflet of the plasma membrane (PM) promoted fusion, and the extent of this promotion increased for the target cells with lower levels of coreceptor expression and upon reduction of the number of fusion-competent Envs. We also found that both single-round infection with HIV-1 Env pseudoviruses and replicative infection with live virus, including HIV-1 infection of human lymphoid tissue ex vivo, depend on PS externalization in the target cells. The dependence is conserved between X4–tropic viruses and R5–tropic viruses, including the physiologically relevant, high CD4–requiring, non-macrophage-tropic R5 virus JR-CSF.
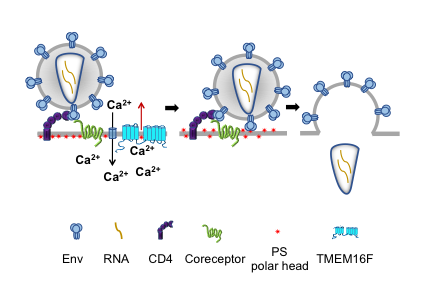
Click image to enlarge.
Figure 1. The fusion stage of HIV-1 entry depends on virus-induced cell-surface exposure of phosphatidylserine.
Our data (Reference 2) indicate that binding of HIV to a cell triggers phosphatidylserine (PS) exposure at the cell surface. This non-apoptotic PS exposure depends on gp120–coreceptor interactions, Ca2+ signaling, and TMEM16F scramblase. Suppression of PS exposure inhibits Env restructuring, viral fusion, and infection.
Our findings suggest that cell-surface PS acts as an important cofactor that promotes the fusogenic restructuring of pre-fusion Env–CD4–coreceptor complexes. A similar promotion of Env–mediated fusion by PS and another anionic lipid, phosphatidylglycerol, suggests the importance of electrostatic interactions rather than specific interactions with the polar head group of PS. We propose that PS at the surface of the target cell lowers the minimal number of coreceptor molecules that need to be engaged by each Env trimer to initiate gp41 refolding. An especially strong fusion promotion by exoPS for target cells with a relatively low density of accessible CCR5 (C-C chemokine receptor type 5) may reflect a stronger dependence of their fusion on gp120 trimer–coreceptor complexes with fewer than three coreceptors. However, we found that fusogenic restructuring of Env depends on cell-surface PS, even for target cells with exceptionally high levels of CCR5 expression, such as TZM-bl cells (HeLa–derived cells expressing CD4 and CCR5 [105 CCR5/cell] and often used in HIV research). The dependence of HIV-1 fusion on surface PS and, by extension, on the signaling triggered by Env–coreceptor interactions was much stronger for the JC10 cells (HeLa–derived cells expressing CD4 and relatively low numbers of CCR5 [about 2 × 103 CCR5/cell], approaching those characteristic for resting peripheral blood lymphocytes [about 600 CCR5/cell]). This finding suggests that PS signaling is essential in physiologically relevant conditions. The uncovered link between HIV-1 infection and PS externalization identifies a bi-directional signaling pathway, in which the classic outside-in signaling through coreceptor triggers, via increase in intracellular Ca2+, inside-out PS externalization mediated by TMEM16F. Given that disrupting the PS externalization pathway suppressed HIV-1 infection, this pathway may present a new target for anti-HIV-1 drugs.
Identification of HAP2 as a gamete protein fusogen
Although all forms of sexual reproduction depend on cell-cell fusion, the machinery mediating sperm-egg fusion has yet to be identified. While HAP2 proteins have been implicated as potential gamete fusogens in Arabidopsis, Chlamydomonas, Tetrahymena, Dictyostelium, and Plasmodium, it was unknown whether the proteins directly mediate gamete fusion, act as an accessory molecule that regulates the activity of an as-yet-unidentified “real” protein fusogen, or are involved in pre-fusion stages, such as signaling or tight adhesion. In our recent study (Reference 1), conducted in collaboration with Benjamin Podbilewicz and Pablo Aguilar, we showed that Arabidopsis HAP2 expression in mammalian cells is sufficient to promote cell-cell hemifusion and fusion. Intriguingly, structural modeling of the HAP2 protein family suggests a striking similarity between HAP2 and the C. elegans fusogens EFF-1 and AFF-1, characterized earlier by us and others, and class II viral fusogens such as dengue protein E and Semliki Forrest virus protein E1. Despite the structural similarity with viral fusogens that merge two membranes when expressed in only one of them, HAP2–mediated fusion (like EFF-1 and AFF-1 fusions) requires the presence of the protein in both fusing membranes. We found earlier that HAP2 expressed in only one of the membranes does not mediate even hemifusion, an early fusion stage that precedes the opening and expansion of a fusion pore. This bilateral requirement can be bypassed by replacing HAP2 with EFF-1 in one of the fusing cells, suggesting that trans HAP2–HAP2 interactions can be substituted by trans HAP2–EFF-1 interactions. Within a month after our paper appeared, two more independent studies, including X-ray crystallographic demonstration of striking similarity between Chlamydomonas HAP2, protein E of dengue virus and ZIKV, and EFF-1, further substantiated the conclusion that HAP2 is indeed the first gamete fusogen.
Additional Funding
- United States-Israel Binational Science Foundation (BSF) grant “Machinery of myoblast fusion” 2015-2019
- Office of Aids Research Award, 2017, 2018
- NICHD Director's Awards, 2016, 2017
Publications
- Valansi C, Moi D, Leikina E, Matveev E, Grana M, Chernomordik LV, Romero H, Aguilar PS, Podbilewicz B. Arabidopsis HAP2/GCS1 is a gamete fusion protein homologous to somatic and viral fusogens. J Cell Biol 2017 216:571-581.
- Zaitseva E, Zaitsev E, Melikov K, Arakelyan A, Marin M, Villasmil R, Margolis LB, Melikyan GB, Chernomordik LV. Fusion stage of HIV-1 entry depends on virus-induced cell surface exposure of phosphatidylserine. Cell Host Microbe 2017 22:99-110.
- Gamage DG, Leikina E, Quinn ME, Ratinov A, Chernomordik LV, Millay DP. Insights into the localization and function of myomaker during myoblast fusion. J Biol Chem 2017 292(42):17272-17289.
Collaborators
- Pablo Aguilar, PhD, Universidad Nacional de San Martin, Buenos Aires, Argentina
- Anush Arakelyan, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Claudia M. Gebert, PhD, Section on Genome Imprinting, NICHD, Bethesda, MD
- Michael M. Kozlov, PhD, Dhabil, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- Leonid Margolis, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Gregory Melikian, PhD, Emory University, Atlanta, GA
- Douglas Millay, PhD, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
- Benjamin Podbilewicz, PhD, Technion-Israel Institute of Technology, Haifa, Israel
- Marian F. Young, PhD, Molecular Biology of Bones and Teeth Section, NIDCR, Bethesda, MD
Contact
For more information, email chernoml@mail.nih.gov or visit irp.nih.gov/pi/leonid-chernomordik.


