Molecular Nature and Functional Role of Dendritic Voltage-Gated Ion Channels

- Dax Hoffman, PhD, Head, Molecular Neurophysiology and Biophysics Section
- Jiahua Hu, PhD, Staff Scientist
- Lin Lin, PhD, Microbiologist
- Ying Liu, MD, Biologist
- Jakob Gutzmann, PhD, Visiting Fellow
- Cole Malloy, PhD, Postdoctoral Fellow
- Jon Murphy, PhD, Postdoctoral Research Associate Program (PRAT) Fellow
- Michelle Hong, BS, Postbaccalaureate Fellow
- Travis Tabor, BS, Postbaccalaureate Fellow
- Adriano Belloiti, BS, Gates Cambridge Scholar
The central nervous system (CNS) underlies all our experiences, actions, emotions, knowledge, and memories. With billions of neurons each firing hundreds of times per second, the complexity of the brain is stunning. To pare down the task of understanding something so complex, our research approach calls for studying the workings of a single central neuron—the pyramidal neuron from the CA1 region of the hippocampus. The hippocampus is essential for long-term memory in humans and is among the first brain regions affected by epilepsy and Alzheimer’s disease. To understand how the hippocampus stores and processes information, we focus on one of its principal cell types, the CA1 pyramidal neuron. Each pyramidal neuron in the CA1 region of the hippocampus receives tens of thousands of inputs onto its dendrites, and it is commonly thought that information is stored by altering the strength of individual synapses (synaptic plasticity). Recent evidence suggests that the regulation of synaptic surface expression of glutamate receptors can, in part, determine synaptic strength. However, the dendrites contain an abundance of ion channels that are involved in receiving, transforming, and relaying information in the dendrites, adding an additional layer of complexity to neuronal information processing.
We found that the A-type potassium channel subunit Kv4.2 is highly expressed in the dendritic regions of CA1 neurons in the hippocampus and, as one of the primary regulators of dendritic excitability, plays a pivotal role in information processing. Kv4.2 is targeted for modulation during the types of plasticity thought to underlie learning and memory. Moreover, we found that the functional expression level of Kv4.2 regulates the subtype expression of NMDA–type glutamate receptors, the predominant molecular devices controlling synaptic plasticity and memory. We are currently following up on these findings with more detailed investigations into the mechanisms of activity-dependent Kv4.2 regulation. In addition, we have begun to investigate the role of dendritic voltage-gated potassium and calcium channels in neuronal development and developmental disorders.
Role of voltage-gated ion channels in synaptic development and disease
Calcium regulation of transient K+ currents
Proteomic and subcellular localization studies suggest that Cav2.3–containing voltage gated calcium channels could be a potential calcium source for a modulatory effect on Kv4.2–mediated A-type K currents (IA) in CA1 hippocampal neurons. Jakob Gutzmann compared wild-type with Cav2.3 knock-out neurons and saw a significant reduction in somatic A-type potassium current in the mutant. We are now analyzing IA from somatic and dendritic attached patch recordings in order to investigate the potential influence of a loss of Cav2.3 on the distinct functional gradient that IA shows along the apical dendrites of wild-type (WT) CA1 pyramidal neurons. To better understand the interplay between calcium and potassium channels that shape CA1 pyramidal cell electrical behavior, we are continuing to characterize Cav2.3 knockout animals.
Isomerase regulation of potassium channel trafficking and function
To identify Kv4.2–binding proteins, Jiahua Hu employed a tandem affinity purification approach (TAP) to isolate the Kv4.2 protein complex from hippocampal neurons. Mass-spectrometry (MS) analysis identified known proteins such as KChIP family members and DPP6/10. The TAP–MS assay also identified an isomerase as a binding partner of Kv4.2. The binding was confirmed by brain co-immunoprecipitation, co-expression in HEK293T cells (a tissue-culture cell line), and peptide pull down in vitro. The isomerase binds to a specific Kv4.2 site, and the association is regulated by neuronal activity and seizure. To determine whether and how the isomerase regulates the trafficking of Kv4.2, we generated bungarotoxin binding site–tagged Kv4.2 at the second extracellular loop for visualizing Kv4.2 in live neurons. In biochemical and electrophysiological assays, the bungarotoxin binding site–tagged Kv4.2 showed similar channel properties to WT Kv4.2. The isomerizing activity may also regulate Kv4.2 binding to its auxiliary subunits. These data suggested that the isomerase plays a role in regulating Kv4.2 function. To further study the physiological function of isomerase and Kv4.2 channel, we generated a knockin mouse in which the isomerase binding site is specifically abolished using Crispr-Cas9 techniques. The mice are viable and appear normal. We are now working on learning- and memory-related behaviors, including novel objective recognition, Morris Water Maze, and fear conditioning.
DPP6 deletion leads to memory impairments in mice.
DPP6 plays an important role as an auxiliary subunit of Kv4, and the DPP6 gene has been associated with neuro-developmental disorders in human. We found that DPP6 deletion leads to behavioral impairments in recognition, spatial learning, and memory, with lower body and brain weights, in adult mice. In addition, we found synaptic structure deficits in neuronal synapses in the hippocampal CA1 region. Using live-image and co-culture assay in neurons, we found that DPP6 induces synapse formation and regulates stabilization.
Loss of regulated Cav2.3 expression in a mouse model of Fragile X syndrome
Fragile X syndrome (FXS) is a severe form of intellectual disability that arises from the loss of the fragile X mental retardation protein (FMRP), an mRNA–binding protein that regulates translation downstream of group I metabotropic glutamate receptors (GpI mGluRs). Loss of FMRP leads to enhanced calcium spiking and neuronal excitability. Former postdoctoral fellow Erin Gray thus sought to explore the possibility that FMRP regulates expression of the dendritic voltage-gated calcium channel Cav2.3.
In our initial studies, we showed that loss of FMRP in mice alters Cav2.3 mRNA levels in both the cortex and hippocampus. Considering that FMRP is an mRNA–binding protein, we performed an RNA immunoprecipitation (RIP) assay and found that immunoprecipitation of FMRP from brain tissue also pulls down Cav2.3 mRNA. The results suggest that FMRP directly binds to Cav2.3 mRNA to regulate its abundance in neurons. FMRP–dependent regulation of Cav2.3 mRNA appears to impact Cav2.3 channel expression, as our previous data showed that FMRP knockout (KO) mice exhibit enhanced expression of Cav2.3 in cortical regions and in the hippocampus. The increase in Cav2.3 expression impacts neuronal physiology; Cav2.3 currents are enhanced in cultured hippocampal neurons isolated from FMRP KO mice compared with wild-type animals. Thus, it appears that FMRP binding normally represses Cav2.3 translation under basal conditions and that loss of FMRP leads to an increase of Cav2.3 protein in the membrane.
We are also investigating the possibility that repression of Cav2.3 expression by FMRP can be regulated by upstream activity of GpI mGluRs. In support of this idea, our previous data showed that stimulation of GpI mGluRs enhances local synaptic translation and expression of Cav2.3 in WT neurons but not in neurons lacking FMRP. To determine whether Cav2.3 expression downstream of GpI mGluR activation plays a role in synaptic plasticity, we induced long-term synaptic depression by stimulating GpI mGluRs (mGluR-LTD) in hippocampal slices from WT and Cav2.3 KO mice. Strikingly, we found that hippocampal slices from Cav2.3 KO mice lacked mGluR-LTD, demonstrating the importance of Cav2.3 in mGluR–dependent synaptic plasticity. Thus, FMRP serves as a key translational regulator of Cav2.3 expression under basal conditions and following activity, and this may be critical for mGluR–dependent forms of plasticity. Loss of regulated Cav2.3 expression could underlie the neuronal hyperactivity and aberrant calcium spiking in FMRP KO mice and contribute to FXS, potentially serving as a novel target for future therapeutic strategies (Figure).
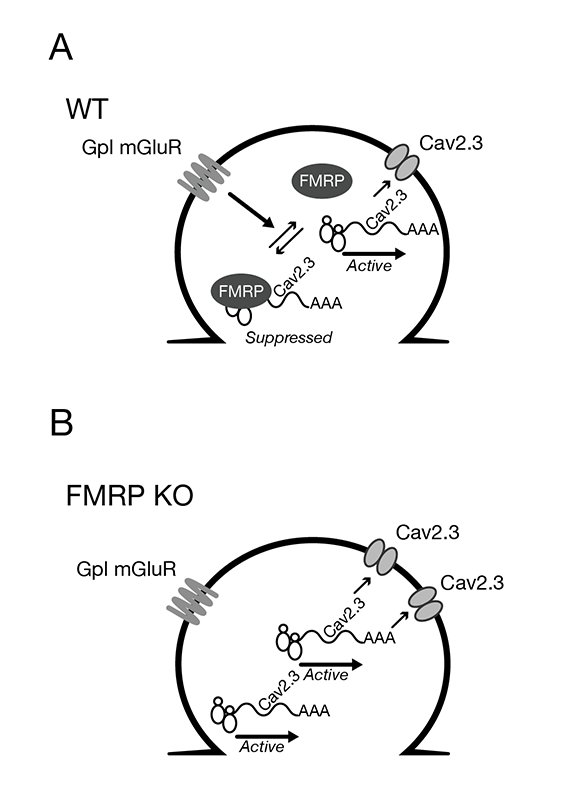
Click image to enlarge.
Disruption of GpI mGluR–dependent Cav2.3 translation in a mouse model of Fragile X syndrome
Working model of FMRP’s regulation of Cav2.3 translation.
A) In WT mice, FMRP binds to Cav2.3 mRNA and functions to suppress its translation in dendrites under basal conditions. Activation of GpI mGluRs relieves FMRP–dependent suppression to allow translation of Cav2.3, and expression of Cav2.3 may be necessary for mGluR-LTD.
B) In the FMRP KO, loss of FMRP–mediated translational suppression of Cav2.3 leads to elevated steady-state levels of Cav2.3 in the plasma membrane. GpI mGluR stimulation can no longer regulate Cav2.3 translation in the absence of FMRP.
Ying Liu showed that Cav2.3 mRNA levels were altered in FMRP KO neurons and that Cav2.3 protein levels were significantly enhanced in the FMRP KO mice. To determine whether Cav2.3 mRNA is one of the targets of FMRP, we performed RNA immunoprecipitation. Our data showed that FMRP interacts with Cav2.3 mRNA in transfected HEK293 cells and in mouse cortex and hippocampus. Our results suggest that FMRP binds to Cav2.3 mRNA directly or indirectly to repress Cav2.3 translation and thus regulates neuronal excitability.
In related work, Jon Murphy is examining whether the dendritic FMRP regulates mRNA trafficking and protein expression of CaV2.3 and KV4.2 in dendrites of hippocampal neurons, using the FMRP KO mouse. Recent progress has centered primarily on analysis of mRNA localization and regulation of total protein translation in neuronal dendrites of WT and FMRP KO mouse neurons. Using fluorescence in situ hybridization to detect mRNAs for CaV2.3 and CaMKII (a serine/threonine-specific kinase regulated by Ca2+) in neurons, we have found that CaMKII mRNA, a known dendritically synthesized protein, has increased abundance throughout the dendritic arbor of FMRP KO mice. FMRP is known to inhibit CaMKII translation through direct binding of CaMKII mRNA, suggesting that either CaMKII mRNA is more highly transcribed in FMRP KO mice, it is no longer sequestered in mRNA–protein complexes where in situ hybridization is inhibited, or both. Conversely, CaV2.3 mRNA signals are low throughout dendrites in both WT and FMRP KO neurons. Studies of Kv4.2 mRNA localization in WT and FMRP KO neurons are ongoing.
Cellular and molecular phenotypes associated with idiopathic autism in iPSC-derived neurons
Idiopathic autism is an early onset neuro-developmental disorder that may result from genetic variation in several genes. We generated induced pluripotent stem cells (iPSCs) from three patients with intrinsic autism as well as from controls; the autistic iPSCs were subsequently differentiated into electrophysiologically active neurons comparable to control cells. Autistic iPSC–derived neurons displayed significant reductions in both Na+ and fast K+ voltage-gated currents but not in Ca2+ and slow K+ voltage-gated currents. The defects accounted for the observation that autistic iPSC–derived neurons reach faster action potential saturation and are slightly more excitable than controls. We found that the amplitude and frequency of spontaneous excitatory post-synaptic currents from autistic neurons were significantly lower than in controls, indicating defects in excitatory synaptic transmission.
Additional Funding
- Postdoctoral Research Associate Program (PRAT) Fellowship (Jon Murphy)
- Gates Cambridge Scholarship (Adriano Bellotti)
Publications
- Hall AM, Throesch BT, Buckingham SC, Markwardt SJ, Peng Y, Wang Q, Hoffman DA, Roberson ED. Tau-dependent Kv4.2 depletion and dendritic hyperexcitability in a mouse model of Alzheimer's disease. J Neurosci 2015 35(15):6221-6230.
- Murase S, Lantz CL, Kim E, Gupta N, Higgins R, Stopfer M, Hoffman DA, Quinlan EM. Matrix metalloproteinase-9 regulates neuronal circuit development and excitability. Mol Neurobiol 2015 53(5):3477-3493.
- Li W, Lee MH, Henderson L, Tyagi R, Bachani M, Steiner J, Campanac E, Hoffman DA, von Geldern G, Johnson K, Maric D, Morris HD, Lentz M, Pak K, Mammen A, Ostrow L, Rothstein J, Nath A. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med 2015 7(307):307ra153.
- Sertedaki A, Markou A, Vlachakis D, Kossida S, Campanac E, Hoffman DA, Sierra ML, Xekouki P, Stratakis CA, Kaltsas G, Piaditis GP, Chrousos GP, Charmandari E. Functional characterization of two novel germline mutations of the KCNJ5 gene in hypertensive patients without primary aldosteronism but with ACTH-dependent aldosterone hypersecretion. Clin Endocrinol (Oxf) 2016 85(6):845-851.
- Liu X, Campanac E, Cheung HH, Ziats MN, Canterel-Thouennon L, Raygada M1, Baxendale V, Pang AL, Yang L, Swedo S, Thurm A, Lee TL, Fung KP, Chan WY, Hoffman DA, Rennert OM. Idiopathic autism: cellular and molecular phenotypes in pluripotent stem cell-derived neurons. Mol Neurobiol 2017 54(6):4507-4523.
Collaborators
- Sachiko Murase, PhD, Laboratory of Molecular Biology, NINDS, Bethesda, MD
- Avindra Nath, MD, Translational Neuroscience Center, NINDS, Bethesda, MD
- Owen M. Rennert, MD, Office of the Scientific Director, NICHD, Bethesda, MD
- Eric Roberson, MD, PhD, University of Alabama, Birmingham, AL
- Constantine A. Stratakis, MD, D(med)Sci, Section on Endocrinology and Genetics, NICHD, Bethesda, MD
- Paul Worley, MD, The Johns Hopkins University, Baltimore, MD
Contact
For more information, email hoffmand@mail.nih.gov or visit http://hoffmanlab.nichd.nih.gov.


