Mechanisms of Disease in Preterm Labor and Complications of Prematurity; Prenatal Diagnosis of Congenital Anomalies

- Roberto Romero, MD, DMedSci, Chief, Perinatology Research Branch
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide. The cost of prematurity in the U.S. alone is estimated to be $26 billion per year. An important goal is to understand the mechanisms of disease responsible for spontaneous preterm birth and fetal injury and to develop methods for the prediction and prevention of preterm birth.
The Perinatology Research Branch (PRB) has proposed that preterm parturition is a syndrome caused by multiple pathologic processes, i.e., that preterm labor is one syndrome but has many causes. The emphasis of our Branch is to study intra-amniotic infection and inflammation, vascular disorders, maternal anti-fetal rejection (chronic inflammatory lesions of the placenta), cervical disease, and a decline in progesterone action. This year, we reported that intra-amniotic inflammation, which affects at least one of every three preterm neonates, is characterized by the activation of amniotic-fluid neutrophils, cells that represent the first line of defense against infection. Using DNA fingerprinting, we determined that amniotic-fluid neutrophils are of fetal origin in cases of preterm labor, maternal origin in cases of clinical chorioamnionitis at term, and mixed origin in patients who have inflammatory processes near term. Moreover, in a series of studies, we were able to demonstrate that neutrophils produce antimicrobial peptides and exhibit the formation of extracellular traps, whereby they immobilize and kill bacteria.
The Branch also studies other obstetrical syndromes that account for the high rate of infant mortality in the United States, including clinical chorioamnionitis, which is the most common infection-related diagnosis in delivery units around the world, as well as meconium aspiration syndrome and amniotic fluid embolism.
Congenital anomalies continue to be a leading cause of perinatal mortality in the U. S. Imaging, a powerful tool for scientific discovery, has changed the practice of obstetrics and maternal-fetal medicine. Imaging with ultrasound allows the definition of fetal anatomy, biometry, growth, and the study of physiologic parameters, such as cardiac function, fetal sleep, and breathing. We invented a new method for the examination of the fetal heart, called fetal intelligent navigation echocardiography (FINE). This year, we reported a major breakthrough: Color Doppler FINE. Color-flow mapping is essential for adequate examination of the fetal heart in those suspected of having congenital anomalies. We demonstrated how Color Doppler FINE can be used to improve the diagnosis of congenital anomalies. The technology has been licensed and is now commercially available to sonographers worldwide.
Although ultrasound is the standard imaging modality in pregnancy, magnetic resonance imaging (MRI) has also been used to characterize fetal anatomy when ultrasound cannot provide definitive diagnostic answers. MRI provides unique information about fetal physiologic parameters (i.e., perfusion, oxygenation, and biochemistry) that are outside the domain of ultrasound. Moreover, MRI can be used to characterize the ontogeny of functional neuro-connectivity, as well as the potential relationship between insults that could alter fetal neuro-development. Given that preterm birth is a leading cause of neuro-developmental disorders, we have used noninvasive methods to interrogate neuro-connectivity. In previous work, we characterized the fetal neuroconnectome using fMRI. This year, we reported a study showing that fetuses subsequently born preterm have a disorder of neuro-connectivity compared with fetuses of the same gestational age subsequently born at term. Neuro-connectivity was reduced in the left hemisphere, close to the pre-language region, providing the first evidence that a disorder of functional connectivity is present in the fetus before birth.
A simple maternal blood test at 24–28 weeks can identify 80% of patients who will subsequently experience a fetal death (Reference 1).
In 2013, fetal death affected 23,595 pregnancies in the U. S., and an estimated 2.6 million third-trimester fetal deaths occurred worldwide. We previously reported that patients who experienced a fetal death had a lower concentration of placental growth factor (PIGF), a higher concentration of soluble endoglin (sENG), as well as a higher expression of soluble vascular endothelial growth factor Receptor-1 (sVERGFR-1) than those with a normal pregnancy after 20 weeks gestation. The objective of this study was to determine whether maternal plasma concentrations of angiogenic and anti-angiogenic factors measured at 24–28 weeks of gestation could predict subsequent fetal death.
We conducted a case-cohort study that included 11 fetal deaths and 829 controls. The placentas of all fetal deaths were examined, and PlGF, sEng, and sVEGFR-1 concentrations were measured between 24–28 weeks of gestation. A positive test was defined as analyte concentrations (or ratios) of lower than 2.5th and 10th centiles [PlGF, PlGF/sVEGFR-1 (angiogenic index-1), and PlGF/sEng)] or greater than 90th and 97.5th centiles (sVEGFR-1 and sEng). The rate of placental lesions consistent with maternal vascular under-perfusion was 33.3% (1/3) among those who had a fetal death at less than 28 weeks and 87.5% (7/8) of those who had this complication at less than or at 28 weeks of gestation. A maternal plasma angiogenic index-1 value lower than the 2.5th centile (0.126) at 24–28 weeks of gestation carries a 29-fold increase in the risk of subsequent fetal death and identifies 55% of subsequent fetal deaths with a false-positive rate of 3.5%. Of note, 61% of women who have a false-positive angiogenic index-1 result will subsequently experience adverse pregnancy outcomes.
In women with intra-amniotic infection and/or inflammation, amniotic fluid neutrophils can be of either fetal or maternal origin (Reference 2).
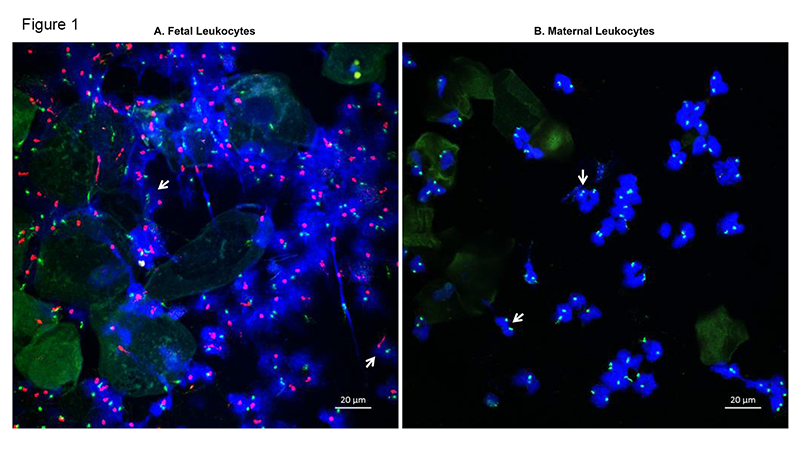
Neutrophils are the most abundant white blood cells in the amniotic cavity of women with intra-amniotic infection and/or inflammation. The current belief is that these neutrophils are of fetal origin. By using DNA fingerprinting, our study provided evidence that, in women with intra-amniotic infection and/or inflammation, amniotic fluid neutrophils can be of either fetal or maternal origin or a mixture of both fetal and maternal. The origin of amniotic fluid neutrophils was confirmed by detecting XY chromosomes (fetal, Figure 1A) or two XX chromosomes (maternal, Figure 1B) in samples collected from women who delivered a male neonate. The findings suggest that both the fetal and maternal innate immune system participate in the mechanisms of host defense against intra-amniotic infection.
Amniotic fluid neutrophils can form neutrophil extracellular traps, or NETs (Reference 3).
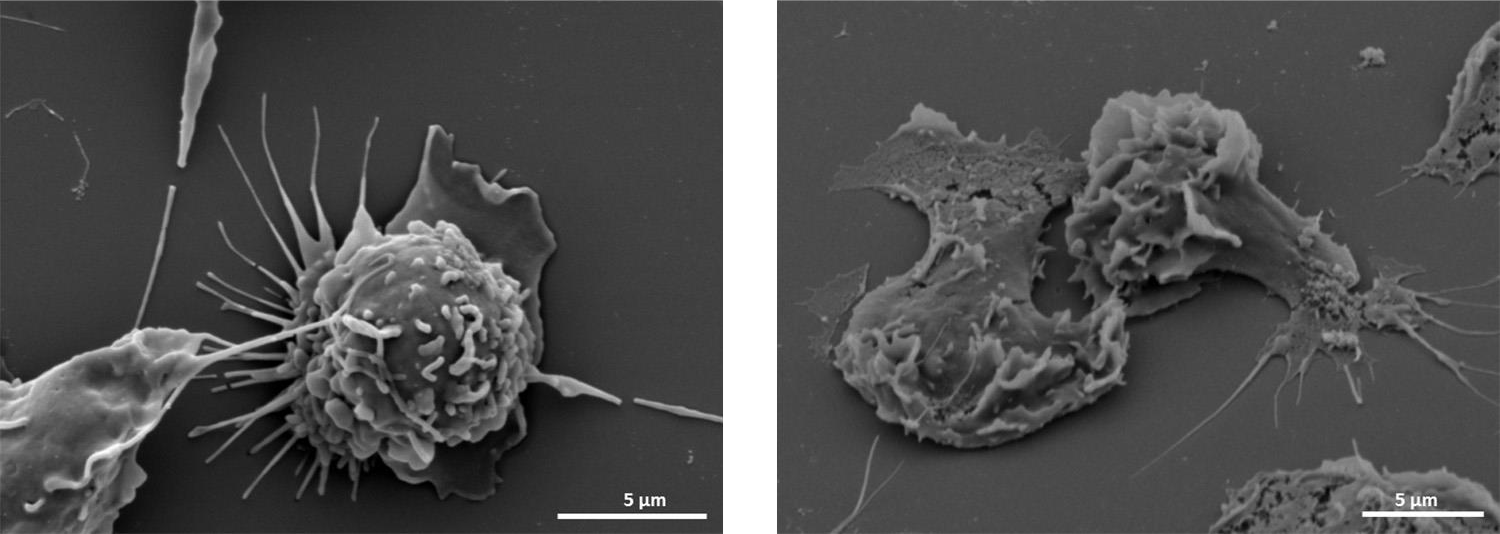
Amniotic fluid neutrophils were thought to participate in the mechanisms of defense against intra-amniotic infection. However, no functional evidence had validated this concept. The study provided in vivo and ex vivo evidence that amniotic fluid neutrophils can form neutrophil extracellular traps, or NETs, a mechanism whereby neutrophils immobilize and kill bacteria invading the amniotic cavity of women with intra-amniotic infection. The findings show that amniotic fluid neutrophils actively participate in the mechanisms of defense against intra-amniotic infection (Figure 2).
Click image to enlarge.
Figure 2.
Scanning electron microscopy. Left: maternal neutrophils in a resting stage with a round shape. Right: an amniotic fluid neutrophil forming a neutrophil extracellular trap with a flattened shape.
Altered functional connectivity in the preterm brain is identifiable before birth (Reference 4).
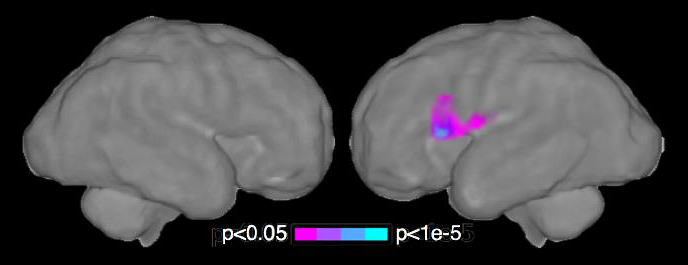
It has been suggested that neurological problems more frequent in those born preterm are expressed prior to birth, but given the technical limitations, this has been difficult to test in humans. We applied novel fetal resting-state functional MRI to measure brain function in 32 human fetuses in utero and found that systems-level neural functional connectivity was diminished in fetuses that would be subsequently born preterm. Neural connectivity was reduced in a left-hemisphere pre-language region, and the degree to which connectivity of this left language region extended to right-hemisphere homologs was positively associated with the time elapsed between fMRI assessment and delivery. The results provide the first evidence that altered functional connectivity in the preterm brain is identifiable before birth and suggest that neuro-developmental disorders associated with preterm birth may result from neurological insults that begin in utero (Figure 3).
Click image to enlarge.
Figure 3.
We have discovered that weaker connections exist in the proto-language network of the preterm brain prior to the complications of early delivery, providing novel insight into the relationship between premature birth and subsequent neurological and behavioral health disorders.
Color and power Doppler combined with Fetal Intelligent Navigation Echocardiography (FINE) to evaluate the fetal heart (Reference 5)
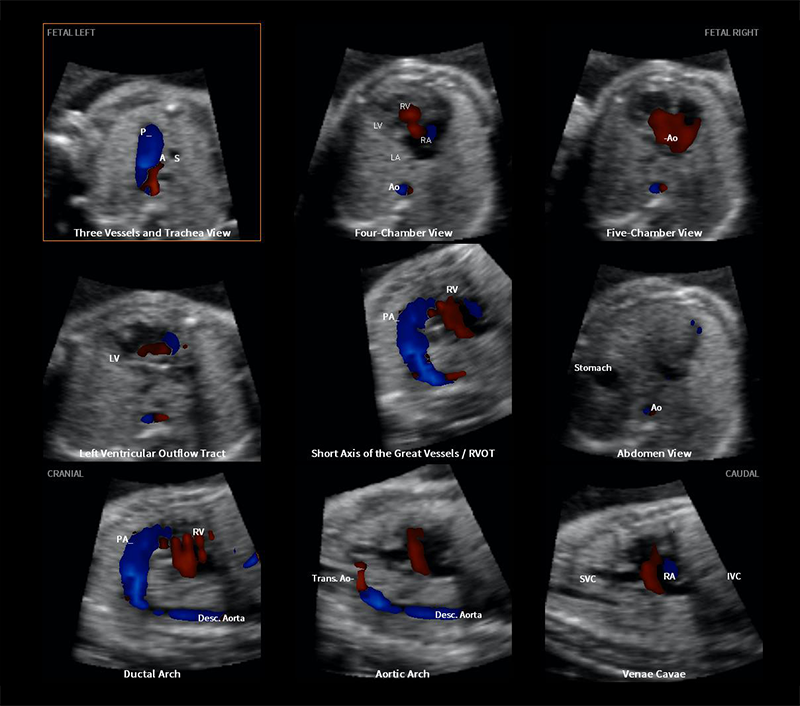
Color Doppler flow mapping is a valuable and integral component of fetal cardiac examination, given that it allows identification of cardiac structures and vasculature, as well as the pattern and direction of blood flow throughout the heart. Indeed, some investigators have recommended that color Doppler should be routinely employed in fetal cardiac screening. In fetuses with congenital heart disease (CHD), color Doppler sonography is essential to identify and characterize abnormal cardiovascular anatomy, flow patterns/disturbances, and cardiac function. For the first time, we combined color and bidirectional power Doppler (S-flow) with Fetal Intelligent Navigation Echocardiography (FINE) to examine the fetal heart. FINE is a novel method invented by our group that interrogates fetal cardiac volume datasets and allows the automatic display of nine standard fetal echocardiography views required to diagnose most cardiac defects. We conducted a prospective cohort study in fetuses with normal hearts or CHD in the second and third trimesters. Sixty cardiac volumes (color Doppler, n=27; S-flow Doppler, n=33) were analyzed using FINE. The rate of successfully generating eight fetal echocardiography views with appropriate color and S-flow Doppler information was 89–100% and 91–100% of cases, respectively, using a combination of diagnostic planes and/or Virtual Intelligent Sonographer Assistance (VIS-Assistance®). However, the success rate for the ninth echocardiography view (i.e., superior and inferior vena cava) was 33% and 30% of cases for color and S-flow Doppler, respectively. In all four cases of congenital heart disease, color Doppler FINE demonstrated evidence of abnormal fetal cardiac anatomy and/or hemodynamic flow. Thus, for the first time, we showed that the FINE method, applied to cardiac volume datasets of normal fetal hearts acquired with color or bidirectional power Doppler information, can successfully generate eight to nine standard fetal echocardiography views (via color or power Doppler) in the second to third trimesters. In addition, for cases of CHD (Figure 4), color Doppler FINE successfully demonstrated abnormal anatomy and/or flow characteristics.
Click image to enlarge.
Figure 4.
This figure of a fetus with hypoplastic left heart and coarctation of the aorta diagnosed prenatally using color Doppler FINE was featured on the cover of Reference 5.
Publications
- Chaiworapongsa T, Romero R, Erez O, Tarca AL, Conde-Agudelo A, Chaemsaithong P, Kim CJ, Kim YM, Kim JS, Yoon BH, Hassan SS, Yeo Y, Korzeniewski SJ. The prediction of fetal death with a simple maternal blood test at 24-28 weeks: a role for angiogenic index-1 (PlGF/sVEGFR-1 ratio). Am J Obstet Gynecol 2017 217(6):682.e1-682.e13.
- Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H, Panaitescu B, Yeo L, Maymon E. Are amniotic fluid neutrophils in women with intra-amniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017 17:31128-31136.
- Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, Jacques SM, Panaitescu B, Garcia-Flores V, Hassan SS. Neutrophil extracellular traps in the amniotic cavity of women with intra-amniotic infection: a New mechanism of host defense. Reprod Sci 2017 24:1139-1153.
- Thomason ME, Scheinost D, Manning JH, Grove LE, Hect J, Marshall N, Hernandez-Andrade E, Berman S, Pappas A, Yeo L, Hassan SS, Constable RT, Ment LR, Romero R. Weak functional connectivity in the human fetal brain prior to preterm birth. Sci Rep 2017 7:39286.
- Yeo L, Romero R. Color and power Doppler combined with Fetal Intelligent Navigation Echocardiography (FINE) to evaluate the fetal heart. Ultrasound Obstet Gynecol 2017 50:476-491.
Collaborators
- Tinnakorn Chaiworapongsa, MD, Wayne State University School of Medicine, Detroit, MI
- Agustin Conde-Agudelo, MD, Wayne State University School of Medicine, Detroit, MI
- Mark Haacke, PhD, Wayne State University School of Medicine, Detroit, MI
- Sonia Hassan, MD, Wayne State University School of Medicine, Detroit, MI
- Edgar Hernandez-Andrade, MD, Wayne State University School of Medicine, Detroit, MI
- Chong-Jai Kim, MD, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
- Leonid Margolis, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Adi L. Tarca, PhD, Wayne State University, Detroit Medical Center, Detroit, MI
- Moriah Thomason, PhD, Wayne State University School of Medicine, Detroit, MI
- Lami Yeo, MD, Wayne State University School of Medicine, Detroit, MI
- Bo Hyun Yoon, MD, PhD, Seoul National University, Seoul, Korea
Contact
For more information, email romeror@mail.nih.gov or visit irp.nih.gov/pi/roberto-romero.