Quantitative Imaging and Tissue Sciences

- Peter J. Basser, PhD, Head, Section on Quantitative Imaging and Tissue Sciences
- Ferenc Horkay, PhD, Staff Scientist
- Dan Benjamini, PhD, Postdoctoral Visiting Fellow
- Beatriz P. Betancourt, PhD, Postdoctoral Intramural Research Training Award Fellow
- Shinjini Kundu, MD, PhD, Postdoctoral Intramural Research Training Award Fellow
- Nathan Hu Williamson, PhD, Postdoctoral Intramural Research Training Award Fellow
- Matan Mussel, PhD, Postdoctoral Visiting Fellow
- Adam Bernstein, MD, Predoctoral Intramural Research Training Award Fellow
- Amber Simmons, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Michal Komlosh, PhD, Contractor funded by the Henry Jackson Foundation-Center for Neuroscience and Regenerative Medicine
- Rea Ravin, PhD, Contractor
In our tissue sciences research, we strive to understand fundamental relationships between function and structure in living tissues, using 'engineered' tissue constructs and tissue analogs. Specifically, we are interested in how microstructure, hierarchical organization, composition, and material properties of tissues affect their biological function or dysfunction. We investigate biological and physical model systems at various length and time scales, performing physical measurements in tandem with developing physical/mathematical models to explain their functional properties and behavior. Experimentally, we use water to probe both equilibrium and dynamic interactions among tissue constituents from nanometers to centimeters and from microseconds to lifetimes. To determine the equilibrium osmo-mechanical properties of well defined model systems, we vary water content or ionic composition systematically. To probe tissue structure and dynamics, we employ atomic force microscopy (AFM), small-angle X-ray scattering (SAXS), small-angle neutron scattering (SANS), static light scattering (SLS), dynamic light scattering (DLS), and one- and two-dimensional nuclear magnetic resonance (NMR) relaxometry and diffusometry. A goal of our basic tissue sciences research is to develop tools that can be translated from bench-based quantitative methodologies to the bedside.
Our tissue sciences activities dovetail with our basic and applied research in quantitative imaging that is intended to generate measurements and maps of intrinsic physical quantities, including diffusivities, relaxivities, or exchange rates, rather than qualitative stains and images conventionally used in neuro-radiology. At a basic level, this work is directed toward making invisible structures and processes visible. Our quantitative imaging group uses knowledge of physics, engineering, applied mathematics, imaging and computer sciences, and insights gleaned from our tissue-sciences research to discover and develop novel imaging biomarkers that sensitively and specifically detect changes in tissue composition, microstructure, or microdynamics. The ultimate translational goal of developing such biomarkers is to assess normal and abnormal development, diagnose childhood diseases and disorders, and characterize degeneration and trauma. Primarily, we use MRI as our imaging modality of choice because it is so well suited to many NICHD mission–critical applications; it is non-invasive, non-ionizing, requires (in most cases) no exogenous contrast agents or dyes, and is generally deemed safe and effective for use with fetuses and children in both clinical and research settings.
A technical objective of our lab has been to transform clinical MRI scanners into scientific instruments capable of producing reproducible, highly accurate, and precise imaging data to measure and map useful imaging quantities for various applications, including single scans, longitudinal and multi-site studies, personalized medicine, genotype/phenotype correlation studies, and for populating imaging databases with high-quality normative data.
In vivo MRI histology
We aim to develop novel next-generation in vivo MRI methods to better understand brain structure and function in normal and abnormal development, disease, degeneration, and trauma. The most mature technology that we invented, developed, and clinically translated is Diffusion Tensor MRI (DTI), by which we measure D, a diffusion tensor of water, voxel-by-voxel within an imaging volume. Information derived from this quantity includes white-matter fiber-tract orientation, the mean-squared distance that water molecules diffuse in each direction, the orientationally averaged mean diffusivity, and other intrinsic scalar (invariant) quantities. These imaging parameters have been used by radiologists and neuroscientists as non-invasive quantitative histological 'stains'. Remarkably, the MRI images are obtained by probing endogenous tissue water in vivo without requiring exogenous contrast agents or dyes. The bulk or orientationally averaged apparent diffusion coefficient (mean ADC) is the most successful and widely used DTI parameter to identify ischemic regions in the brain during acute stroke and to follow cancer patients' response to therapy. Our measures of diffusion anisotropy (e.g., fractional anisotropy or FA) are used universally to follow changes in normally and abnormally developing white matter, including dysmyelination and demyelination and many other applications of brain white matter visualization. Our group also pioneered the use of fiber direction–encoded color (DEC) maps to display the orientation of the main association, projection, and commissural white matter pathways in the brain. To assess anatomical connectivity among various cortical and deep-brain gray-matter areas, we also proposed and developed DTI "Streamline" Tractography. Collectively, these advances helped inspire several large federally-funded research initiatives, such as the NIH Human Connectome Project (HCP).
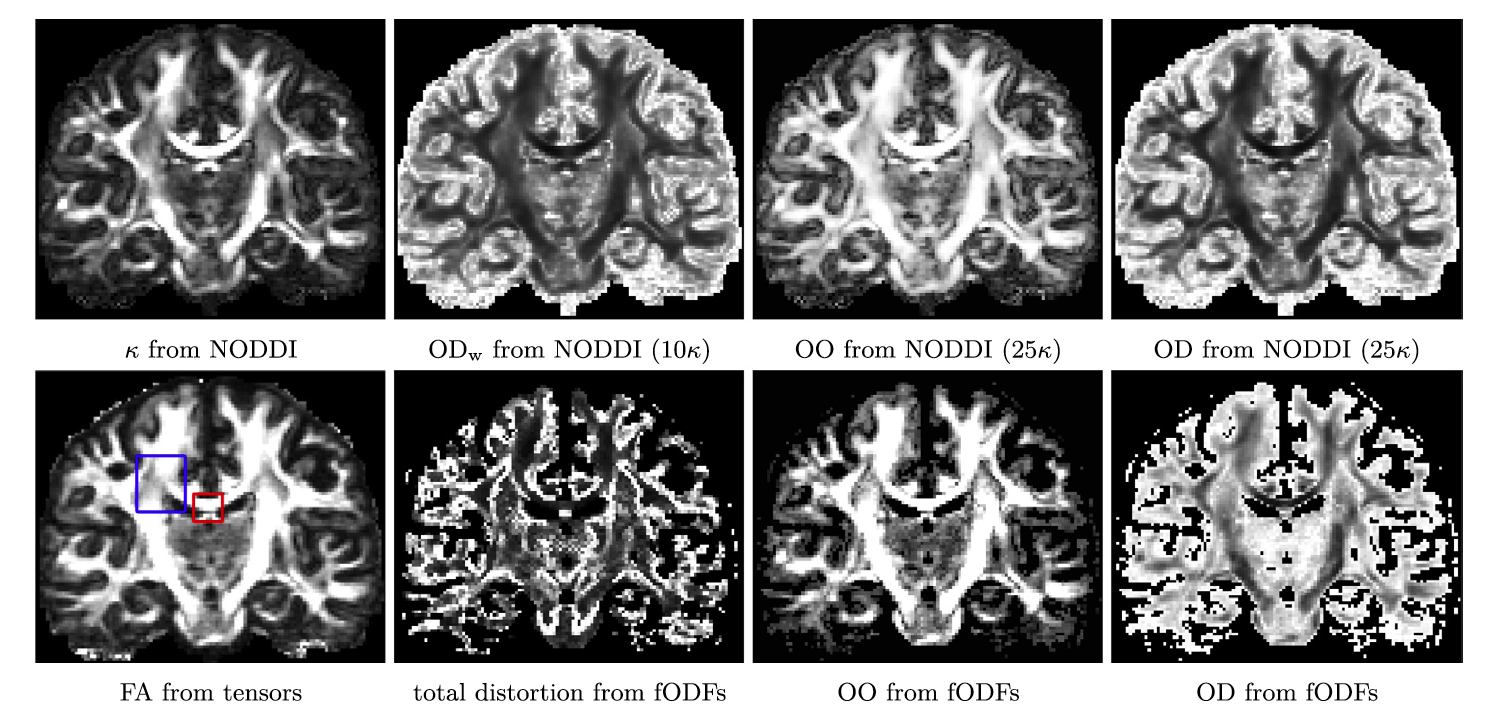
Click image to view.
Figure 1. New stains of white matter pathway morphology derived from differential geometric features
Our new method produces geometric features of white matter pathways, such as the way they splay, bend, and twist as they traverse the brain. First row: Results for multi-shell Human Connectome Project (HCP) data. Second row: DTI and NNSD results for HCP data, where various measures of orientational distortion are calculated.
More recently, we invented and developed a family of advanced in vivo diffusion MRI methods to measure fine-scale microstructural features of axons and fascicles, which otherwise could only be measured using laborious ex vivo histological methods. We have been developing efficient means for performing "k and q-space MRI" in the living human brain, such as "Mean Apparent Propagator" (MAP) MRI. This approach detects subtle microstructural and architectural features in both gray and white matter at micron-scale resolution, several orders of magnitude smaller than the typical MRI voxel. MAP-MRI also subsumes DTI, as well as providing a bevy of new in vivo quantitative 'stains' to measure and map. We also developed a family of diffusion MRI methods to 'drill down into the voxel' and measure features such as average axon diameter (AAD) and axon-diameter distribution (ADD) within and along large white-matter pathways, dubbing them CHARMED and AxCaliber MRI, respectively. After careful validation studies, we reported the first in vivo measurement of ADDs within the rodent corpus callosum. The ADD is functionally important, given that axon diameter is a determinant of axon or nerve conduction velocity and therefore the rate at which information flows along white matter pathways, helping to determine the delays or latencies between and among different brain areas. We then developed a companion mathematical theory to explain the observed ADDs in different fascicles, suggesting that they represent a trade-off between maximizing information flow and minimizing metabolic demands. We also developed novel multiple pulsed-field gradient (mPFG) methods and demonstrated their feasibility for use in vivo on conventional clinical MRI scanners as a further means to extract quantitative features to measure and map in the central nervous system (CNS). The methods can also provide an independent measurement of the AAD and other features of cell size and shape.
Although gray matter appears featureless in DTI brain maps, its microstructure and architecture are rich and varied throughout the brain, not only along the brain's cortical surface, but also within and among its various cortical layers and within deep gray-matter regions. To target this tissue, we have been developing several noninvasive, in vivo methods to measure unique features of cortical gray matter microstructure and architecture that are currently invisible in conventional MRI. One of our long-term goals is to 'parcellate' or segment the cerebral cortex in vivo into its approximately 500 distinct cyto-architechtonic areas. To this end, we are developing advanced MRI sequences to probe correlations among microscopic displacements of water molecules in the neuropil as well as sophisticated mathematical models to infer distinguishing microstructural and morphological features of gray matter. Within the past year, we pioneered and developed several promising two-dimensional MRI relaxometry, exchange, and diffusometry methods, which we are using to study water mobility and exchange in gray matter and white matter. We believe these be promising in identifying inflammation and redistribution of tissue water in brain parenchyma, as well.
In general, we are continuing to develop translationally oriented methods to follow normal and abnormal development and aid in the diagnosis of various diseases and disorders of the brain, noninvasively and in vivo.
Quantitative fetal and pediatric MRI
MRI is considered safer than X-ray–based methods, such as computed tomography (CT), for scanning fetuses, infants and children. However, clinical MRI data still lack the quantitative character of CT data. Clinical MRI relies upon the acquisition of 'weighted images,' whose contrast is affected by many factors, some intrinsic to the tissue and some dependent on the details of the experiment and experimental design. The diagnostic utility of conventional MRI for many neurological disorders is unquestionable. However, the scope of conventional MRI applications is limited to revealing either gross morphological features or focal abnormalities, which result in regional differences in signal intensities within a given tissue. Clinical MRI also often lacks biological specificity. Although quantification per se does not ensure improved specificity, it is nonetheless necessary for developing robust and reliable imaging 'biomarkers.' In particular, MRI assessment of normal brain development and developmental disorders has benefiting greatly from the introduction of 'quantitative' clinical MRI techniques, with which one obtains maps of meaningful intrinsic physical quantities or chemical variables that can be measured in physical units and compared among different tissue regions, in individual subjects, and in longitudinal and cross-sectional studies. Quantitative MRI methods, such as DTI, also increase sensitivity, providing a basis for monitoring subtle changes that occur, e.g., during the progression or remission of disease, by comparing measurements in a single subject with normative values acquired in a healthy population. Quantitative MRI methods should continue to advance precision imaging studies, whereby MRI phenotypic data can be meaningfully combined with a subject's genotype.
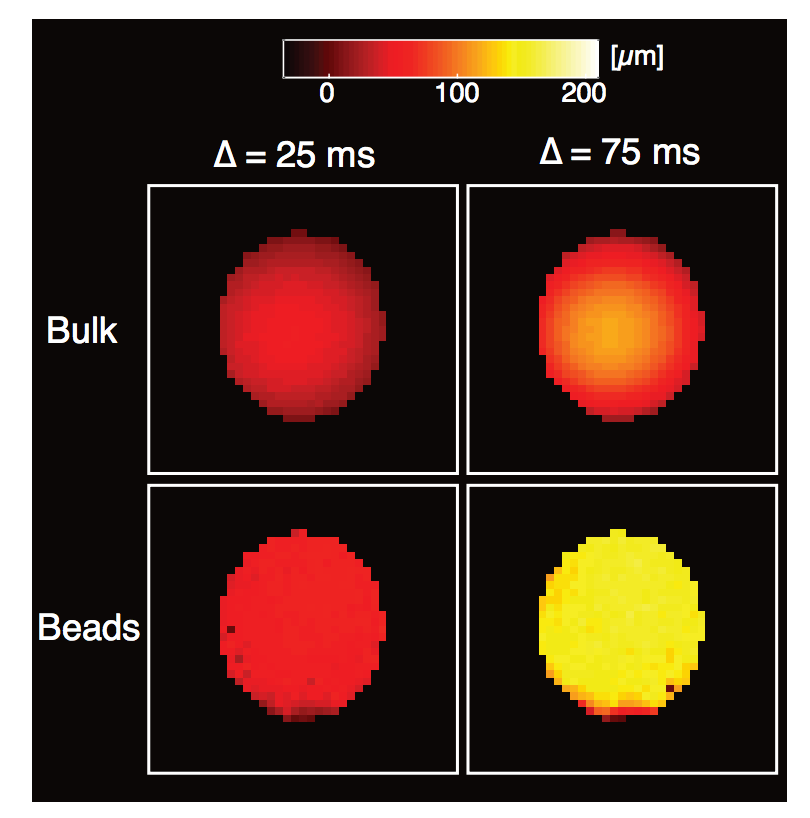
Click image to view.
Figure 2. Development of novel net displacement imaging method capable of measuring and mapping very slow flows
The method is designed to one day measure very slow flows of CSF and other body fluids, which currently cannot be measured with conventional MRI methods. Average displacement images of the different flow regimes observed in our novel pack-bed MRI phantom obtained under different experimental conditions. Note the signature Poiseuille flow profile of the bulk water images and the overall higher displacement of water transported through the bead pack compared with bulk water.
Our group is known for carrying out key clinical studies that utilize novel quantitative MRI acquisition and analysis methods and whose aim is to improve accuracy and reproducibility in diagnosis and to detect and follow normal and abnormal development. One example is the NIH Study of Normal Brain Development, jointly sponsored by a consortium of four NIH Institutes (NICHD, NIMH, NINDS, and NIDA). This multi-center effort, initiated in 1998, was intended to advance our understanding of normal brain development in typical healthy children and adolescents. Remarkably, the Brain Development Cooperative Group (http://www.brain-child.org), created by this forward-looking mechanism, is still active, publishing numerous papers each year, primarily by mining these rich data, many of which we processed, vetted, and uploaded. Our role in this interdisciplinary project was as the DTI Data-Processing Center (DPC). We have uploaded all admissible DTI data collected from this project into a database accessible to interested investigators, which are made publicly available through the National Database for Autism Research (NDAR; https://nda.nih.gov). In collaboration with Carlo Pierpaoli, we continue to support and help update and disseminate this processing and analysis software, called "TORTOISE," which can be downloaded from http://www.tortoisedti.org.
Our involvement in traumatic brain injury (TBI) research, particularly in detecting mild TBI (mTBI), has continued to expand owing to its high relevance to the NICHD mission. TBI is an acute problem in the pediatric population, but it also affects and afflicts men and women in the military. DTI provides essential information for the diagnosis of TBI and has potential as an important tool for the assessment of structural damage in the brain. For clinical applications, however, reliable imaging protocols are needed. Part of our work has been to develop a robust DTI data–processing pipeline in order to improve the accuracy and reproducibility of DTI findings, in collaboration with scientists at the Center for Neuroscience and Regenerative Medicine (CNRM) and for the larger clinical and scientific community involved in TBI research. To this end, we are adding new modules to our existing state-of-the-art DTI data-processing pipeline, as well as tools to permit calibration of DTI experiments, using our novel polyvinyl pyrrolidone (PVP) polymer–based diffusion MRI phantom that we developed, patented, and are disseminating to numerous clinical sites worldwide.
To permit analysis of novel MRI data as well as to develop new clinical and biological applications of quantitative MRI, we need to continue to create and disseminate a mathematical, statistical, and image sciences–based infrastructure. To date, we have developed algorithms that generate a continuous, smooth approximation of the discrete, noisy, measured DTI field data so as to reduce noise and allow us to follow fiber tracts more reliably. We proposed a novel Gaussian distribution for tensor-valued random variables that we use in designing optimal DTI experiments and interpreting their results. In tandem, we developed non-parametric empirical (e.g., Bootstrap) methods to determine the statistical distribution of DTI–derived quantities in order to study, for example, the inherent variability and reliability of computed white-matter fiber-tract trajectories. Such parametric and non-parametric statistical methods enable us to apply powerful hypothesis tests to assess the statistical significance of findings in a wide range of important biological and clinical applications that are currently being tested using ad hoc statistical methods. We are also developing novel methods to register or warp different brain volumes and to generate group-average data or atlases from various subject populations based on methods such the Kullback-Leibler divergence. Recently, our group developed methods for studying the reproducibility and reliability of different tractography methods, given their widespread use to assess anatomical connections between different brain regions in vivo. In the area of artifact remediation and correction, we pioneered methods to correct for subject motion and for artifacts caused by induced eddy-current and echo-planar imaging (EPI) distortion. However, much work remains to be done in order to address and remedy MRI artifacts to permit one to draw statistically significant inferences from clinical DTI data, obtained in longitudinal and multi-center studies, particularly single-subject studies.
Biopolymer physics: water-ion-biopolymer interactions
A major focus of this basic research project is to understand the effect of ion-water-biopolymer interactions. These are ubiquitous in biology, ranging from the movement of water and ions across channels, to the self-assembly of aberrant proteins into nanofibers in neuro-degenerative processes, such as Creutzfeldt-Jakob disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE). Despite their importance and prevalence, however, little is understood about the physical underpinnings of these interactions, which underlie a myriad of biological behaviors.
To address this dearth in our understanding of ion-water-biopolymer interactions, we have developed a comprehensive multi-scale experimental framework to study them by combining macroscopic techniques (e.g., osmotic swelling pressure measurements, mechanical measurements) with high-resolution scattering methods, such as NMR and neutron and light scattering experiments. Swelling pressure measurements provide information on the overall thermodynamic response, while high-resolution scattering methods allow us to investigate biopolymers at the molecular and supramolecular levels and to quantify the effect of ion concentration, ion valance, pH, and temperature on the structure and macroscopic (thermodynamic) properties of the tissue. We also apply computational techniques to model water-ion-biopolymer interactions in these systems.
We have been able to apply our understanding of water-polymer interactions to produce novel diffusion MRI phantoms that we use to calibrate MRI scanners, specifically to assure the quality of the imaging data and to assess scanner performance on an on-going basis. Our recently issued U.S. Patent for a "Phantom for diffusion MRI imaging" is enabling quantitative diffusion MRI studies to be performed at many different sites. The polymer consisting of the phantom polyvinylpyrrolidone (PVP) has ideal properties for this demanding application. It is chemically and thermally stable, has a long shelf life, is safe and non-toxic, can be shipped from site to site, and has stable diffusion and relaxation properties. Colleagues at NIST in Boulder, Colorado, have incorporated our PVP polymer into NIST's own diffusion MRI standard. The technology is also being promulgated commercially e.g., by https://hpd-online.com/more-hpd-products.
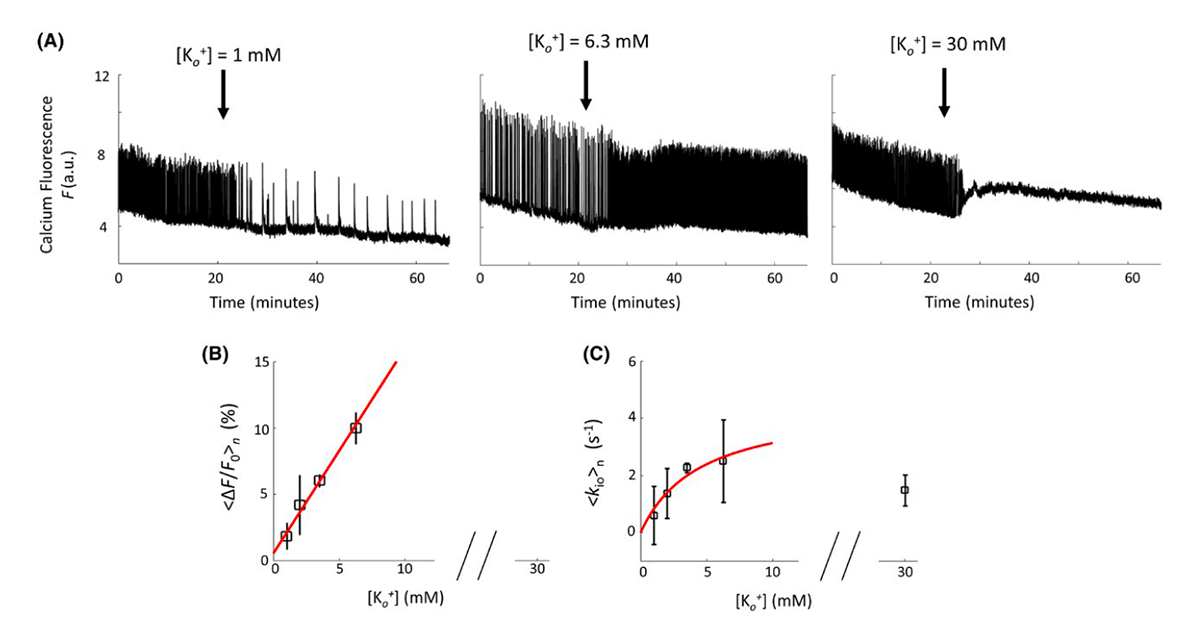
Click image to view.
Figure 3. Demonstration of MRI-based method for measuring increased water exchange associated with neuronal excitation
The figure shows changes in neuronal activity and accompanying water cycling rate measured in organotypic brain cultures. Neuronal activity is measured by monitoring the change in fluorescence of an optical indicator/dye, whereas MRI is used to monitor changes in water exchange across neuronal membranes.
Measuring and mapping functional properties of extracellular matrix
Our first goal is to understand and quantify the interactions among the major macromolecular components of extracellular matrix (ECM), which give rise to its unique functional properties. ECM is present in every tissue and performs a key role in determining normal and abnormal tissue and organ function. Specifically, we are studying interactions among the primary ECM components, namely collagen, proteoglycans (PG), water, and ions, which govern ECM's macroscopic mechanical and transport properties, using cartilage as a model system. The biomechanical behavior of cartilage and other ECMs reflects molecular composition and microstructure, which change during development, disease, degeneration, and aging. Understanding the basis of important functional properties of cartilage, particularly its load-bearing and lubricating abilities, requires an array of experimental techniques that probe a wide range of relevant length and time scales. Understanding the physical and chemical mechanisms affecting cartilage swelling (hydration) is essential to predicting these properties, which are mainly governed by osmotic and electrostatic forces. This knowledge can inform tissue-engineering or regenerative-medicine strategies to grow, repair, and reintegrate replacement cartilage. To obtain a self-consistent physical picture of tissue structure/function relationships, we measure various physical/chemical properties of ECM tissues and tissue analogs at different length- and time-scales, using a variety of complementary static and dynamic experimental techniques, e.g., osmometry, SANS, SAXS, neutron spin-echo (NSE), SLS, DLS, AFM, and fluorescence correlation spectroscopy (FCS).
Controlled tissue hydration provides a direct means of determining the viability and load-bearing ability of cartilage ECM. Previously, we designed and built a tissue micro-osmometer to perform high-precision swelling pressure measurements on small tissue samples (less than 1 microgram) as a function of the water activity (vapor pressure). We make osmotic pressure measurements to determine how the individual components of cartilage ECM (e.g., aggrecan, hyaluronic acid [HA], and collagen) contribute to the total load-bearing capacity of the tissue. We demonstrated that aggrecan–HA aggregates self-assemble into microgels, contributing to improved dimensional stability and the tissue's lubricating ability. We also found that aggrecan is highly insensitive to changes in the ionic environment, particularly to the concentration of calcium ions, which is critically important in maintaining the tissue's mechanical integrity in high Ca2+ environments and in allowing aggrecan to serve as a calcium ion reservoir in cartilage and bone.
We recently began developing a new biomimetic model of cartilage ECM, consisting of polyacrylic acid (PAA) microgel particles dispersed and embedded within a polyvinyl alcohol (PVA) gel matrix. In this novel system, PAA mimics the behavior of proteoglycan (i.e., HA and aggrecan) microgel assemblies, while PVA mimics the role of the collagen network. The PVA/PAA biomimetic model system reproduces not only the shape of the cartilage swelling pressure curves, but also the numerical values reported for healthy and osteoarthritic human cartilage samples. Systematic studies made on model composite hydrogels is expected to provide invaluable insights into the effects of various factors (matrix stiffness, swelling pressure, fixed-charge density, synovial fluid composition, etc.) on the macroscopic mechanical/swelling properties, and ultimately the load-bearing and lubricating ability of cartilage. Similar studies cannot be obtained from measurements made on biological tissues because their composition, structure, and physical properties cannot be independently and systematically varied as they can in these synthetic model polymer composites.
The resistance of tissue to external loads is determined by its osmotic modulus. Therefore, maps of the osmotic modulus are particularly useful for characterizing the load-bearing properties of cartilage. We developed a method that utilizes the precise scanning capabilities of the AFM to generate compliance maps, from which relevant elastic properties can be extracted. We then combined AFM with tissue micro-osmometry to generate elastic and osmotic modulus maps of cartilage.
We have begun translating this critical tissue-science understanding of the structure/function relationships of components of ECM to develop and design novel non-invasive MR imaging methods, with the aim of inferring ECM composition, patency, and functional properties in vivo. Our goal is to use MRI for early diagnosis of cartilage and other ECM diseases, as well as to provide a means for following normal and abnormal development of the ECM. This challenging project entails making 'invisible' components of ECM, (e.g., collagen and PGs) 'visible' and then using our understanding of biopolymer interactions to predict functional properties, such as tissue load-bearing ability. One major obstacle is that water molecules bound to immobile species (e.g., collagen) are largely invisible to conventional MRI approach. However, magnetization transfer (MT) MRI (as well as other methods) make it possible to detect the bound protons indirectly by transferring their magnetization to the free water surrounding them. It also makes it possible to obtain an estimate of the collagen content in tissue. In a pilot study with collaborators Uzi Eliav and Ed Mertz, we applied the new MT MRI method to determine the concentration and distribution of the main macromolecular constituents in bovine femoral-head cartilage samples. The results obtained by the MT MRI method are qualitatively consistent with those obtained by histological techniques, such as high-definition infrared (HDIRI) spectroscopic imaging. This work was originally aided by a DIR Director's Award that we received with our collaborators Sergey Leikin and Edward Mertz.
Patents
- Benjamini D, Basser PJ. Multi-dimensional spectroscopic NMR and MRI using marginal distributions. USPTO Patent No. WO2018031942-A1 2018.
- Horkay F, Pierpaoli C, Basser PJ. Phantom for diffusion MRI imaging. USPTO 10,078,124 2018.
- Benjamini D, Basser P. Multi-dimensional spectroscopic NMR and MRI using marginal distributions. Patent No. WO2018031942-A1, filed August 11, 2017, and issued February 15, 2018.
Additional Funding
- "Characterizing brain microstructure in patients with mTBI using Mean Apparent Propagator (MAP) MRI." HJF Award Number: 308049-8.01-60855, (CNRM-89-3895), which is under the joint auspices of the NIH, DoD, CNRM, and USUHS.
- "Development of Bench and Pre-Clinical MRI Methods to Assess Glymphatic Clearance in the Living Brain." 308811-4.01-60855, (CNRM-89-9237)
- "In vivo Brain Network Latency Mapping." NIH BRAIN Initiative grant 1-R24-MH-109068-01
Publications
- Benjamini D, Basser PJ. Magnetic resonance microdynamic imaging reveals distinct tissue microenvironments. Neuroimage 2017;163:183-196.
- Bai R, Springer CS, Plenz D, Basser PJ. Fast, Na+/K+ pump driven, steady-state transcytolemmal water exchange in neuronal tissue: a study of rat brain cortical cultures. Magn Reson Med 2018;79:3207–3217.
- Avram AV, Sarlls JE, Hutchinson E, Basser PJ. Efficient experimental designs for isotropic generalized diffusion tensor MRI (IGDTI). Magn Reson Med 2018;79:180–194.
- Chandran PL, Dimitriadis E, Mertz E, Horkay F. Microscale mapping of matrix elasticity of neonatal mouse joint cartilage: an approach to extracting bulk elasticity of soft matter with surface roughness. Soft Matter 2018;14(15):2879-2892.
- Horkay F, Basser PJ, Hecht AM, Geissler E. Microstructure and dynamic properties of aggrecan assemblies. MRS Adv 2018;3(28):1589-1595.
Collaborators
- Alexandru Avram, PhD, NIBIB, Bethesda, MD
- Ruliang Bai, PhD, Zhejiang University, Hangzhou, China
- Emilios Dimitriadis, PhD, Division of Bioengineering and Physical Science, NIBIB, Bethesda, MD
- Uzi Eliav, PhD, Tel Aviv University, Tel Aviv, Israel
- Dario Gasbarra, PhD, University of Helsinki, Helsinki, Finland
- Erik Geissler, PhD, CNRS, Université Joseph Fourier de Grenoble, Grenoble, France
- Mark Hallett, MD, PhD, Human Motor Control Section, NINDS, Bethesda, MD
- Iren Horkayne-Szakaly, MD, Joint Pathology Center, Armed Forces Institute of Pathology, Washington, DC
- Sergey Leikin, PhD, Section on Physical Biochemistry, NICHD, Bethesda, MD
- Pedro Miranda, PhD, Universidade de Lisboa, Lisbon, Portugal
- Edward L. Mertz, PhD, Section on Physical Biochemistry, NICHD, Bethesda, MD
- Gil Navon, PhD, Tel Aviv University, Tel Aviv, Israel
- Uri Nevo, PhD, Tel Aviv University, Tel Aviv, Israel
- Sinisa Pajevic, PhD, Mathematical and Statistical Computing Laboratory, CIT, NIH, Bethesda, MD
- Carlo Pierpaoli, MD, PhD, Section on Quantitative Medical Imaging, NIBIB, Bethesda, MD
- Dietmar Plenz, PhD, Section on Critical Brain Dynamics, NIMH, Bethesda, MD
- Tom Pohida, MS, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Randall Pursley, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Evren Özarslan, PhD, Linköping University, Linköping, Sweden
- Bradley Roth, PhD, Oakland University, Rochester, MI
- Joelle Sarlls, PhD, In Vivo NMR Center, NINDS, Bethesda, MD
- Charles C. Springer, PhD, Oregon Health and Sciences University, Portland, OR
- Brain Development Cooperative Group, Various
Contact
For more information, email pjbasser@helix.nih.gov or visit http://sqits.nichd.nih.gov.


