Neuronal Circuits Controlling Behavior: Genetic Analysis in Zebrafish

- Harold Burgess, PhD, Head, Section on Behavioral Neurogenetics
- Tripti Gupta, PhD, Staff Scientist
- Ashwin A. Bhandiwad, PhD, Postdoctoral Fellow
- Eric J. Horstick, PhD, Postdoctoral Fellow
- Abhignya Subedi, PhD, Postdoctoral Fellow
- Victoria Heigh, BSc, Postbaccalaureate Fellow
- Hussain Ather, BSc, Postbaccalaureate Fellow
- Jennifer L. Sinclair, MSc, Zebrafish Technician
Our goal is to understand how, under diverse environmental contexts, the nervous system selects appropriate behavioral actions in a way that best satisfies internal motivational objectives. We use the zebrafish as a model because, in the larval stage, its brain exhibits the basic architecture of the vertebrate brain but is much less complex than the mammalian brain. Despite the relative simplicity of their nervous system, larvae have a sophisticated repertoire of sensory-guided and internally driven behaviors. Furthermore, the optical clarity of the embryo facilitates visualization of individual neurons and their manipulation with genetic techniques. Behavior in larvae is innate and therefore exhibits minimal variability between fish. Subtle alterations in behavior can therefore be robustly scored, making it possible to quickly assess the contribution of identified neurons to a variety of motor behaviors.
We focus on two aspects of behavioral regulation: the mechanisms by which sensory context regulates behavioral decisions and the pathways that sustain changes in behavioral state. In addition, we are developing a suite of genetic tools and behavioral assays to probe the nexus between neuronal function and behavior at single-cell resolution. The neuronal connections that allow the brain to integrate sensory and internal-state information are established through genetic interactions during development. We aim to identify genes and neurons that are required for the functional development of such connections. In vertebrates, neuronal circuits situated in the brainstem form the core of the locomotor control network and are responsible for balance, posture, motor control, and arousal. Accordingly, many neurological disorders stem from abnormal formation or function of brainstem circuits. Insights into the function of brainstem circuits in health and disease have come from genetic manipulation of neurons in zebrafish larvae in combination with computational analysis of larval behavior.
Molecular identification of neurons that mediate prepulse inhibition
Startle responses are rapid reflexes that are triggered by sudden sensory stimuli and which help animals defend against or escape from potentially threatening stimuli. In both fish and mammals, startle responses are initiated by giant reticulospinal neurons in the medulla, which receive short-latency sensory input from diverse sensory modalities. Although highly stereotyped, startle responses are nevertheless modulated by sensory context and behavioral state and are therefore an excellent system in which to understand how such information is integrated for behavioral choice. In mammals, including humans, the startle response to a strong auditory stimulus can be inhibited by pre-exposure to a weak acoustic ‘prepulse.’ This form of startle modulation, termed prepulse inhibition (PPI), is diminished in several neurological conditions. Vibrational stimuli trigger rapid-escape swims in zebrafish, which are mediated by giant reticulospinal neurons, in a manner similar to the central neurons controlling startle responses in mammals. Escape swims are suppressed by pre-exposure to a prepulse, allowing us to apply the powerful suite of genetic tools available in zebrafish to identify neurons that mediate prepulse inhibition.
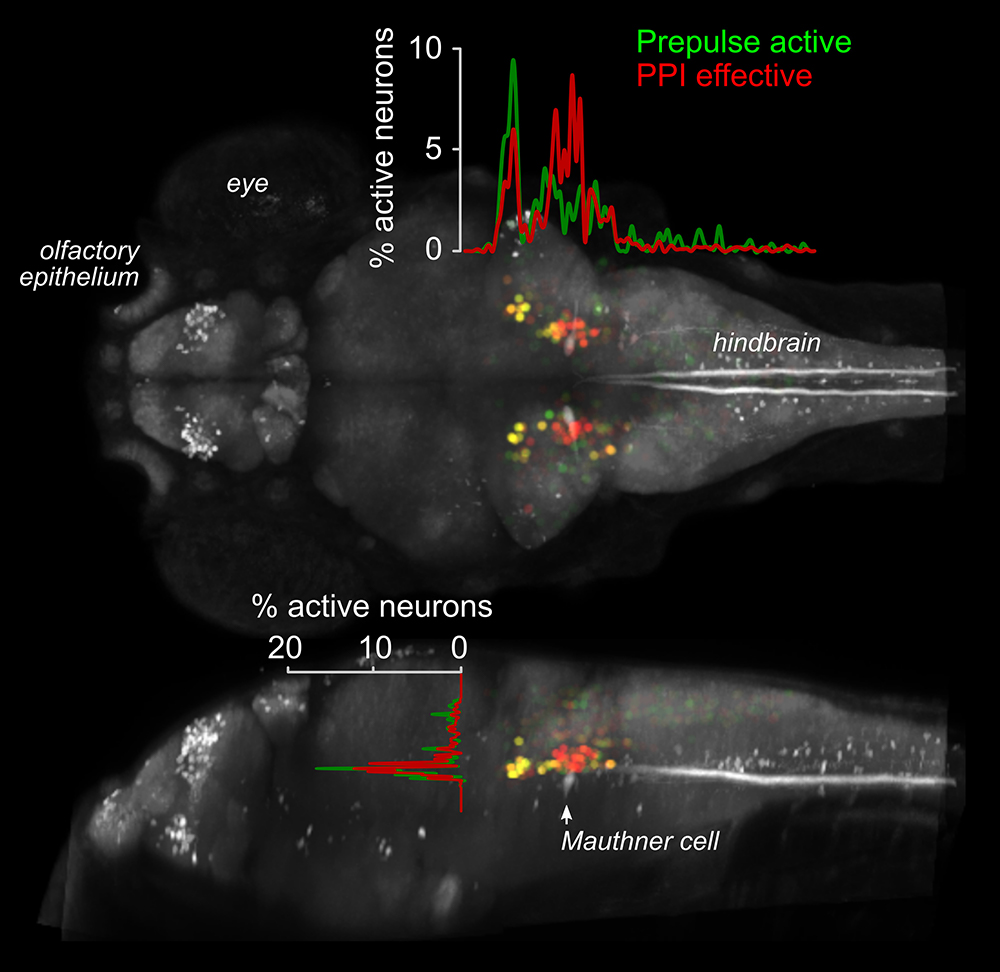
To identify a transgenic zebrafish line that genetically labels neurons required for PPI, we screened a library of neuron-specific Gal4–enhancer trap lines marking distinct populations of neurons in the brain, by ablating the neurons in each enhancer trap line before testing for PPI. The transgenic line y252 labels a discrete population of neurons in the hindbrain whose ablation or optogenetic inhibition eliminates PPI. The neurons labeled in y252 are specified by the transcription factor Gsx1. We found that Gsx1–knockout mice showed a strong reduction in PPI, suggesting that a conserved circuit involving Gsx1–specified neurons mediates PPI across vertebrate species. To identify the precise subset of Gsx1 neurons that mediate PPI, we then used volumetric calcium imaging to simultaneously visualize the activity in tens of thousands of Gsx1 neurons during PPI (Figure 1). The method enabled us to locate a specific brain region that contains Gsx1 neurons whose activity correlates strongly with behavioral PPI. To determine where such neurons are causally related to PPI, we optogenetically activated them before probing fish with a startle stimulus. Controlled firing of Gsx1 neurons was able to reproduce the effect of a prepulse. We next visualized the morphology and projections of these neurons in order to reveal how they connect to the core startle circuit. For this, we developed a new method that exploits the low efficiency of B3 recombinase in zebrafish to achieve stochastic labeling of isolated neurons in cells that co-express Gal4 and Cre recombinase. We found a specific subset of Gsx1 neurons that use the neurotransmitter glutamate and that project to an area of the lateral dendrite of the Mauthner cell, the command neuron for startle responses in fish, that receives auditory input. By directly visualizing neurotransmitter release from the auditory nerve, we discovered that acoustic information to the Mauthner cell is selectively depressed during PPI, while transmission to other brain regions is spared, which indicates that a key mechanism for PPI is presynaptic inhibition [Reference 1]. Given that PPI is abnormal in neuro-psychiatric disorders with developmental origins, including schizophrenia and autism, our work will help identify and probe fundamental defects in circuitry that are abnormal in these conditions.
Click image to view.
Figure 1. Activity imaging during prepulse inhibition
Activity map for neurons that show elevated activity in response to a weak auditory stimulus (Prepulse active, green), and on trials where the prepulse inhibits responses to a subsequent startle stimulus (PPI effective, red), superimposed on a standard zebrafish reference brain (grey). Neurons that show activity correlated with behavioral prepulse inhibition are clustered in rhombomere 4, adjacent to the command neuron for startle responses, the Mauthner cell (indicated).
Tools for decoding neuronal circuits
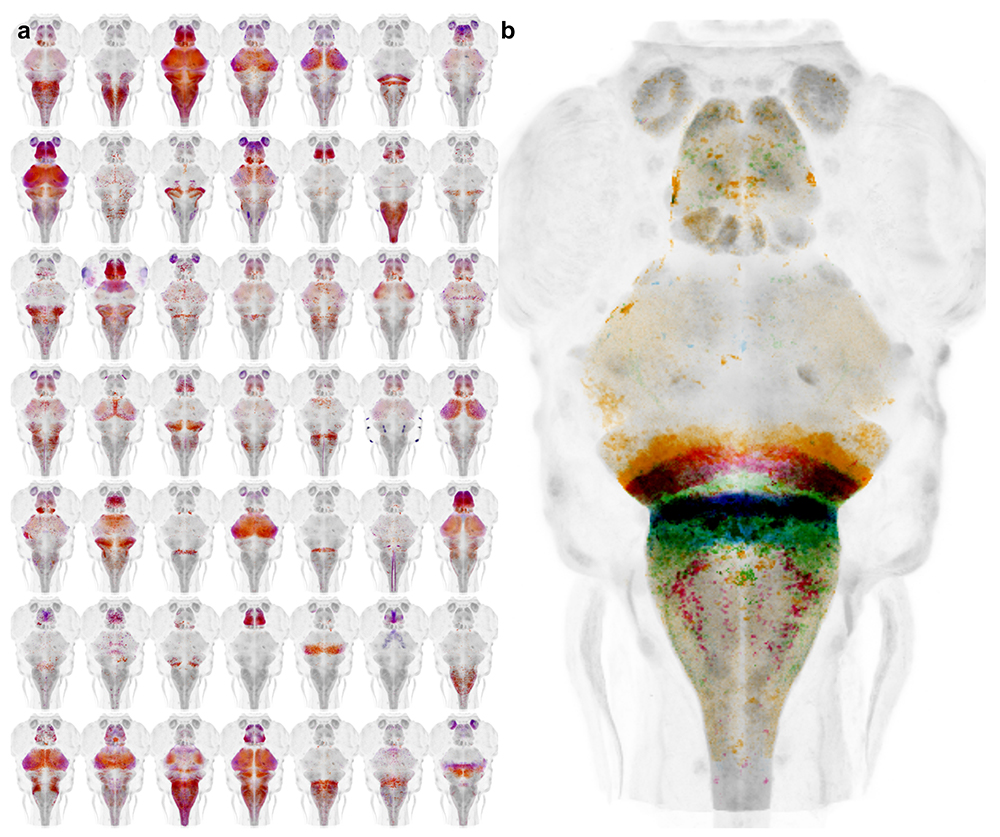
During our work to identify the neuronal basis for prepulse inhibition, we realized that existing transgenic tools did not allow manipulation of small groups of neurons with a high degree of specificity. We developed a system to leverage the thousands of Gal4 lines already available in zebrafish, by generating a new library of Cre enhancer trap lines with restricted patterns of expression within the brain (Figure 2a). The lines can be used 'intersectionally' to restrict the pattern of expression with Gal4 domains, allowing small clusters of neurons to be targeted. During our studies on prepulse inhibition, we used a subset of the lines to ablate Gsx1 neurons within specific hindbrain domains to determine which specific neurons are required for prepulse inhibition (Figure 2b).
Click image to view.
Figure 2. Cre enhancer trap lines for neural circuit analysis in zebrafish
a. Whole brain projections of the expression pattern for new Cre transgenic lines
b. Cre lines with expression in specific rhombomeres enable the function of neurons in different hindbrain territories to be parsed.
A unique feature of brain imaging in zebrafish is the ability to visualize the total architecture of the brain while simultaneously recording the position and morphology of every constituent labeled neuron. Brain registration techniques enable data from several individual zebrafish to be quantitatively compared, so that experiments can systematically address the functional contributions of neurons across the entire brain. However, a challenge for deformable brain registration in larval zebrafish is to achieve highly precise global registration, without severely distorting the shape of individual cells. We calibrated parameters for the symmetric diffeomorphic normalization algorithm in ANTs (Advanced Normalization Tools) and found that it was possible to align larval zebrafish brains to a precision of around 1 cell diameter without sacrificing cell morphology [Reference 2].
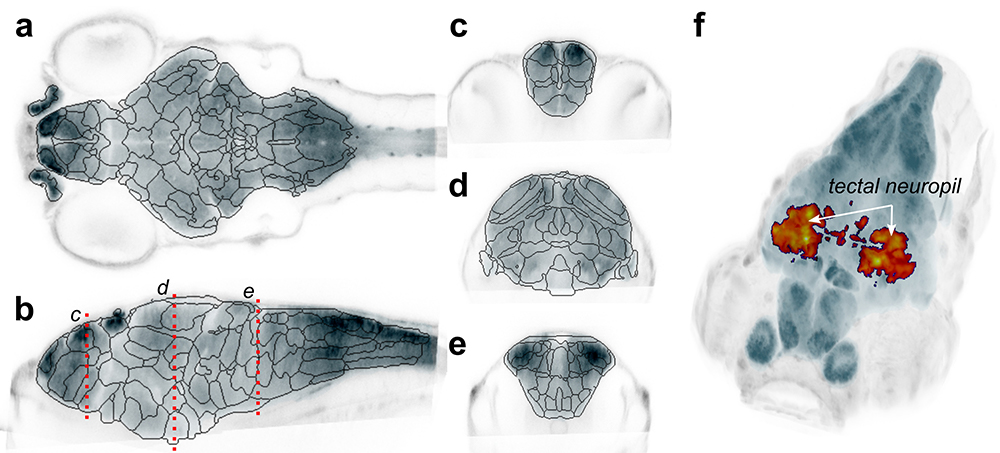
We postulated that the very high precision of alignment may enable statistically robust whole-brain analysis of neuronal composition and morphology in zebrafish models of neurological disorders. For this, we first developed a new computational method to automatically segment the larval zebrafish brain into 180 neuroanatomical regions, based on gene expression patterns (Figure 3a). We then generated new software, Cobraz, that uses those regions to identify changes in brain size, neurotransmitter distribution, or local morphology in confocal images of genetic mutant zebrafish larvae. We validated our algorithm by comparing embryos injected with the atoh7 morpholino to wild-type siblings. Loss of atoh7 is known to eliminate retino-fugal projections. Indeed, we found that the optic tectum, the major retino-recipient area, was significantly smaller in morphants (Figure 3b) [Reference 3]. In collaboration with the Dawid laboratory, we used the software to assess brain development in zebrafish kctd15 mutants, which show a growth reduction but no overt change in brain structure. Mutant larvae showed a significant loss of the torus lateralis, a region known to produce growth hormone–releasing hormone [Reference 4]. The technique can be applied to almost any zebrafish neuro-developmental model, thus enabling robust and quantitative detection of subtle changes in brain structure or composition.
Click image to view.
Figure 3. Unbiased analysis of brain structure and composition in mutant models
a–e. Computationally derived neuroanatomical regions based on cluster analysis of more than 200 transgene expression patterns. Images are single slices through a reference brain in horizontal (a), sagittal (b), and coronal (c–e) planes. Planes for c–e are indicated in b.
f. Deformational field analysis of atoh7 morphant larvae. Heat map reveals regions that are smaller in mutants, statistically significant after correction for comparison of more than 5.3 M voxels. A large cluster is detected within the neuropil region of the optic tectum (indicated), corresponding to the loss of projections from retinal ganglion cells.
Neural mechanisms for behavioral state control
Over the course of the day, motivational goals change in response to both internal and external cues. At any given moment, an individual's current behavioral state strongly influences decisions on how to interact with the environment. A major goal in neuroscience is to identify the neural systems that maintain short-term behavioral states and to determine how they interact with central mechanisms for behavioral choice. In zebrafish, loss of illumination triggers a short-term behavioral state in which larvae show heightened locomotor activity. We previously demonstrated that light-sensitive neurons in the hypothalamus trigger this state of hyperactivity. However, the experiments were performed on larvae confined to small shallow chambers, making it difficult to characterize the underlying behavioral state. By recording larval movement in large-volume arenas, we found that the response to loss of illumination is partly a light-search behavior. For the first two minutes in the dark, larval swim patterns are similar to a behavior characterized as area-restricted search across species, with high fractal dimension and repeated turning behavior. Larvae quickly locate and swim toward a light-spot if activated during this period. If no light-spot is found, the locomotor profile transitions to a remote-search behavior, in which larvae efficiently locate illuminated regions that are not visible in the original environment. Using a G0 cas9/crispr screen, we found that the transition between these two behavioral states requires the activity of otpa neurons, including opn4a–expressing deep brain photoreceptors [Reference 5].
Additional Funding
- US Israel Binational Fund 2013433 (2014): Ongoing, with Yoav Gothilf, Tel Aviv University
- DIR Director's Award, NICHD (2018-2019) with Dr. David Clark, NICHD
- DIR Director's Award, NICHD (2018-2019) with Dr. Karl Pfeifer, NICHD
Publications
- Tabor KM, Smith TS, Brown M, Bergeron SA, Briggman KL, Burgess HA. Presynaptic inhibition selectively gates auditory transmission to the brainstem startle circuit. Curr Biol 2018;28(16):2527-2535.
- Marquart GD, Tabor KM, Horstick EJ, Brown M, Burgess HA. High precision registration between zebrafish brain atlases using symmetric diffeomorphic normalization. GigaScience 2017;6:1-15.
- Gupta T, Marquart GD, Horstick EJ, Tabor KM, Pajevic S, Burgess HA. Morphometric analysis and neuroanatomical mapping of the zebrafish brain. Methods 2018;150:49-62.
- Heffer A, Marquart G, Beck A, Saleem N, Burgess HA, Dawid IB. Generation and characterization of Kctd15 mutations in zebrafish. PLoS One 2017;12:e0189162.
- Horstick EJ, Bayleyen Y, Sinclair JL, Burgess HA. Search strategy is regulated by somatostatin signaling and deep brain photoreceptors in zebrafish. BMC Biol 2017;15(1):4.
Collaborators
- Kevin Briggman, PhD, Circuit Dynamics and Connectivity Unit, NINDS, Bethesda, MD
- Igor Dawid, PhD, Section on Developmental Biology, NICHD, Bethesda, MD
- Yoav Gothilf, PhD, Tel-Aviv University, Tel-Aviv, Israel
- Sinisa Pajevic, PhD, Mathematical and Statistical Computing Lab, CIT, NIH, Bethesda, MD
Contact
For more information, email haroldburgess@mail.nih.gov or visit http://ubn.nichd.nih.gov.


