Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Aloa L. Dams, PhD, Visiting Fellow
- Yuta Moshimaru, PhD, Visiting Fellow
- Rafael M. Previde, PhD, Visiting Fellow
- Milos B. Rokic, PhD, Visiting Fellow
- Kosara Smiljanic, PhD, Visiting Fellow
- Kai Wang, PhD, Visiting Fellow
- Qing Chen, MD, Guest Researcher
- Marko Jovic, PhD, Guest Researcher
We investigate cellular signaling cascades, gene expression, and hormone secretion in hypothalamic and pituitary cells, with a special emphasis on the interactions between plasma-membrane electrical events and receptor-controlled pathways. Specifically, we are addressing how these neuroendocrine cells use ion channels and G protein–coupled receptors as signaling platforms to efficiently process information. To this end, we characterize both native and recombinant receptors and channels that have been cloned from neuroendocrine cells. In the past, our work has focused on the role of inositol-trisphosphate receptors in the oscillatory calcium release of pituitary cells, the mechanism of periodic activation of these channels, and the complex mode of synchronization of calcium release from intracellular stores with electrical activity of cells. We also characterized voltage-gated channels expressed in neuroendocrine cells, the cell type–specific patterns of electrical activity and channels involved, the physiological relevance of such activity, and the crosstalk between G protein–coupled receptors and ion channels. More recently, we characterized ligand-gated receptor channels expressed in pituitary cells, including the ATP–gated P2X receptor channels. Our current work focuses on age-, sex-, and tissue structure–specific signaling, transcription and secretion in the pituitary gland, the heterogeneity of secretory pituitary cells reflecting their embryonal and postnatal genesis, and cell type–specific exocytic pathways. We are also studying how the structural features of P2X receptors relate to the channels' functions and how plasma membrane receptors and the intracellular signaling milieu affect channel activity.
Ligand-gated receptor channels: regulation and function
Former Visiting Fellow Claudio Coddou started two projects on the regulation and function of P2X receptor channels (P2XRs). The work was recently completed and published. The first project focused on the kinetics of P2X2R desensitization. The channels exhibited a slow desensitization during the initial ATP application and a progressive, calcium-dependent increase in rates of desensitization during repetitive stimulation. He observed the pattern in whole-cell recordings from cells expressing recombinant and native P2X2R, and it was termed use-dependent desensitization (UDD). However, no desensitization was observed in perforated-patched cells and in two-electrode voltage clamped oocytes. Addition of ATP, but not of ATPS or GTP, also abolished UDD, whereas intracellular injection of apyrase facilitated receptor desensitization. Experiments with injection of alkaline phosphatase or addition of staurosporine and ATP in the intracellular solution suggested a role for a phosphorylation-dephosphorylation in receptor desensitization. Mutation of residues that are potential phosphorylation sites identified a critical role of the S363 residue in the intracellular ATP action. The findings indicate that intracellular calcium and ATP have opposing effects on P2X2R gating: calcium allosterically facilitates receptor desensitization, and ATP covalently prevents the action of calcium. Single-cell measurements further revealed that intracellular calcium remains elevated after washout in P2X2R–expressing cells, and the blockade of mitochondrial sodium/calcium exchanger lowers calcium concentrations during washout periods to basal levels, suggesting a role of mitochondria in this process. Therefore, the metabolic state of the cell can influence P2X2R gating [Reference 1].
Coddou's second project focused on the role of cyclin-dependent kinase 5 (Cdk5) in P2X2R gating. A putative Cdk5 phosphorylation site is present in the full-size variant P2X2aR, which is absent from the splice variant P2X2bR. We found an interaction between P2X2aR and Cdk5/p35 by co-immunofluorescence when expressed in HEK293 culture cells. We also found that threonine phosphorylation was significantly higher in HEK293 cells co-expressing P2X2a and p35 than in cells expressing only P2X2aR. Moreover, P2X2a–derived peptides encompassing the Cdk5 consensus motif were phosphorylated by Cdk5/p35. Whole-cell patch-clamp recordings indicated a delay in development of UDD of P2X2aR, but not of P2X2bR, in cells co-expressing these receptors and p35. In Xenopus oocytes, P2X2aR showed a slower UDD than in HEK293 cells, and Cdk5 activation prevented this effect. A similar effect was found in P2X2a/3R heteromeric channels. The P2X2a-T372A receptor mutant was resistant to UDD. By co-localization using immunofluorescence in primary culture of nociceptive neurons, we observed similar distribution between P2X2aR and Cdk5/p35 in endogenous cells. Moreover, co-immunoprecipitation experiments showed an interaction between Cdk5 and P2X2aR in mouse trigeminal ganglia. Endogenous P2X2aR–mediated currents in PC12 cells and P2X2/3R–mediated increases of intracellular calcium in trigeminal neurons were Cdk5–dependent, given that inhibition with the cyclin-dependent kinase inhibitor roscovitine accelerated the desensitization kinetics of these responses. The results indicate that the P2X2aR is a novel target for Cdk5–mediated phosphorylation, which might play important physiological roles in, for example, pain signaling [Reference 2].
In general, the functions of anterior pituitary cells are controlled by two major groups of hypothalamic and intrapituitary ligands: one exclusively acts on G protein–coupled receptors and the other activates both G protein–coupled receptors and ligand-gated receptor channels. The second group of ligands operate as neurotransmitters in neuronal cells, and their receptors are termed as neurotransmitter receptors. Most information about pituitary neurotransmitter receptors was obtained from secretory studies, RT-PCR (reverse transcriptase-polymerase chain reaction) analyses of mRNA expression, and immunohistochemical and biochemical analyses, which were all performed using a mixed population of pituitary cells. However, recent electrophysiological and imaging experiments characterized GABA–, acetylcholine-, and ATP–activated receptors and channels in single pituitary cell types, expanding this picture and revealing surprising differences in the receptors' and channels' expression between subtypes of secretory cells and between native and immortalized pituitary cells. Recently, we summarized the current knowledge on the electrophysiological and pharmacological properties of these receptors and their roles in calcium signaling and calcium-controlled hormone secretion [Reference 3]. Figure 1 summarizes the current knowledge and hypotheses regarding the expression and role of these channels in secretory pituitary cells.
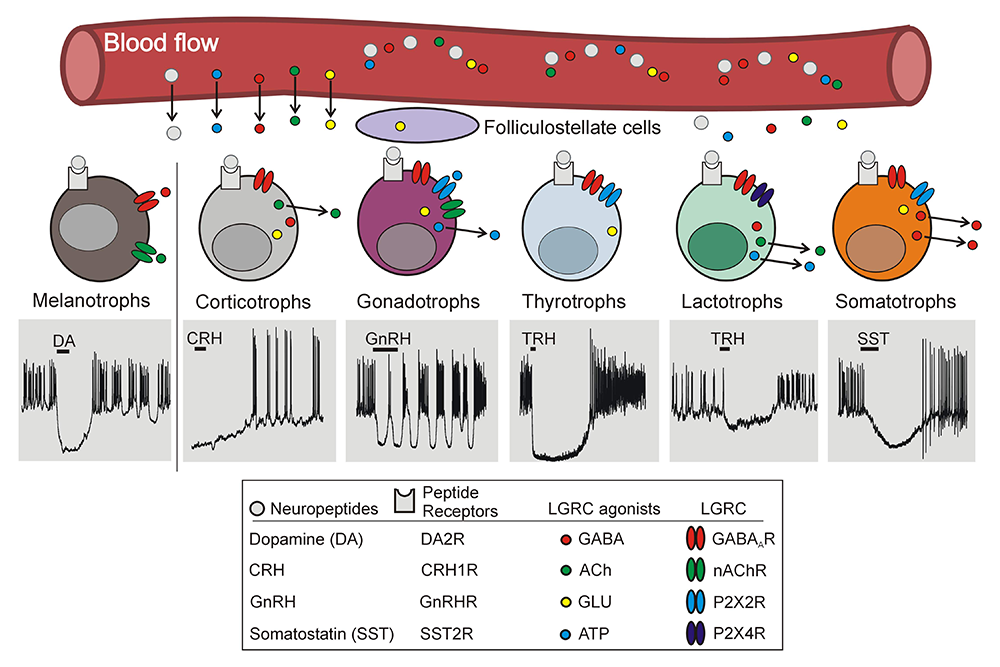
Click image to view.
Figure 1. Cell type–specific expression of neurotransmitters and their receptors in pituitary gland
Ligand-gated receptor channel agonists (GABA, ACh, GLU, and ATP) are synthesized in hypothalamus and transported by the hypophysial portal blood flow to anterior pituitary cells and/or synthesized and released by anterior pituitary cells. Evidence confirming functional expression of their receptors in different pituitary cell subtypes was obtained after their identification. Secretory anterior pituitary cells other than lactotrophs, as well as folliculostellate cells, also synthetize glutamate, but functional iGluRs (ionotropic glutamate receptors) have not yet been electrophysiologically detected in any type of pituitary cells. Gray area indicates membrane potential values from -100 mV to +50 mV (Y-axis) and duration of recordings (melanotrophs: 30 s; corticotrophs: 80 s; gonadotrophs and somatotrophs: 40 s; thyrotrophs: 75 s; and lactotrophs: 95 s [X-axis]).
Voltage-gated channels: regulation and function
In collaboration with Patrick Fletcher and Arthur Sherman, we examined common and diverse elements of ion channels and receptors underlying electrical activity in six major secretory pituitary cells: corticotrophs, melanotrophs, gonadotrophs, thyrotrophs, somatotrophs, and lactotrophs. The cell types are all electrically excitable, and voltage-gated calcium influx is the major trigger for their hormone secretion. Along with hormone intracellular content, G protein–coupled receptor and ion channel expression can also be considered as defining cell-type identity. While many aspects of the developmental and activity-dependent regulation of hormone and G protein–coupled receptor expression have been elucidated, much less is known about the regulation of the ion channels needed for excitation-secretion coupling in these cells. We compared the spontaneous and receptor-controlled patterns of electrical signaling among endocrine pituitary cell types, including insights gained from mathematical modeling. We argue that a common set of ionic currents unites these cells, while differential expression of another subset of ionic currents could underlie cell type–specific patterns. Using a generic mathematical model, we supported these ideas, showing that the model reproduces many of the observed features of pituitary electrical signaling. Mapping our observations to the developmental lineage suggests possible modes of regulation that may give rise to mature pituitary cell types [Reference 4].
Role of phosphatidylinositol 4-kinase in cellular functions
Tamás Balla’s group recently reported that sciatic nerves of mice lacking phosphatidylinositol 4-kinase alpha (PI4KA) in Schwann cells show substantially reduced myelin thickness, with grave consequences for nerve conductivity and motor functions. However, prolonged inhibition of PI4KA in immortalized mouse Schwann cells failed to lower plasma-membrane phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) levels or PI 3-kinase (PI3K) activation, in spite of large reductions in plasma-membrane PI4P levels. Instead, it caused rearrangements of the actin cytoskeleton, which was also observed in sciatic nerves of knockout animals. These and other studies define a role for PI4KA in myelin formation primarily affecting metabolism of key phospholipids and the actin cytoskeleton. Our group contributed to this work with sciatic nerve histochemistry and immunohistochemistry [Reference 5].
Our ongoing collaborative studies with Tamás Balla’s group focus on the role of PI4K in pituitary cell functions. The preliminary data, obtained with the phosphatidylinositol kinase inhibitor wortmannin (Wm), at a concentration that inhibits both PI3Ks and PI4Ks (10 µM), indicated that high basal growth hormone release was not affected, and neither was basal and GnRH–stimulated follicle-stimulating hormone (FSH) secretion. In contrast, high basal prolactin (PRL) release was inhibited in a time-dependent manner by 10 µM Wm. Basal luteinizing hormone (LH) release was very low and not affected by Wm, but the sustained GnRH–stimulated secretion was abolished without affecting the initial peak response. Thyrotropin-releasing hormone (TRH)–induced early and sustained PRL release was both inhibited by Wm. Also, concentration-dependent effects of Wm on basal PRL release in static cultures showed enhancement at 100 nM and inhibition at 10 µM, consistent with opposing contribution of Wm–sensitive PI kinases to control of action-potential secretion coupling in lactotrophs. These results clearly show that Wm affects regulated but not constitutive exocytosis in pituitary gonadotrophs and lactotrophs, two cell types critical for control of reproductive functions. Based on the inhibitory potency curves, the involvement, in lactotrophs, of type III PI4Ks and/or PI3Ks of low Wm sensitivity was implicated in inhibition of PRL and LH secretion, while PI3Ks of high Vm sensitivity (presumably the class-I of these enzymes) in facilitation of action-potential secretion-coupling. Therefore, analysis of the roles of phosphoinositides in secretory functions of gonadotrophs and lactotrophs is warranted. It is important to stress that all our preliminary experiments were performed with pituitaries from females, and that studies have to be extended to males. The first goal of ongoing investigations is to pharmacologically identify the PI kinases involved in LH and PRL secretion. The subsequent goal of ongoing investigations is to explore which step(s) of the complex sequences of the signaling and/or secretion pathways is affected and by which phosphoinositide. We are also progressing in development of appropriate conditional knockout mice models for in vitro and in vivo studies.
Publications
- Rokic MB, Castro P, Leiva-Salcedo E, Tomic M, Stojilkovic SS, Coddou C. Opposing roles of calcium and intracellular ATP on gating of the purinergic P2X2 receptor channel. Intern J Mol Sci 2018;19:1161.
- Coddou C, Sandoval R, Castro P, Lazcano P, Hevia MJ, Rokic M, Hall B, Terse A, Gonzalez-Billault C, Kulkarni AB, Stojilkovic SS, Utreras E. Cyclin-dependent kinase 5 modulates the P2X2a receptor channel gating through phosphorylation of C-terminal threonine 372. Pain 2017;158:2155-2168.
- Fletcher PA, Sherman A, Stojilkovic SS. Common and diverse elements of ion channels and receptors underlying electrical activity in endocrine pituitary cells. Mol Cell Endocrinol 2018;463:49-64.
- Zemkova H, Stojilkovic SS. Neurotransmitter receptors as signaling platforms in anterior pituitary cells. Mol Cell Endocrinol 2018;463:49-64.
- Alvarez-Prats A, Bjelobaba I, Aldworth Z, Baba T, Abebe D, Kim YJ, Stojilkovic SS, Stopfer M, Balla T. Schwann-cell-specific deletion of phosphatidylinositol 4-kinase alpha causes aberrant myelination. Cell Rep 2018;23:2881-2890.
Collaborators
- Tamás Balla, MD, PhD, Section on Molecular Signal Transduction, NICHD, Bethesda, MD
- Claudio Coddou, PhD, Faculty of Medicine, Universidad Católica del Norte, Coquimbo, Chile
- Patrick A. Fletcher, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Hana Zemková, PhD, Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic
Contact
For more information, email stankos@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx or www.nichd.nih.gov/research/atNICHD/Investigators/stojilkovic.


