Receptors and Actions of Peptide Hormones and Regulatory Proteins in Endocrine Mechanisms

- Maria L. Dufau, MD, PhD, Head, Section on Molecular Endocrinology
- Raghuveer Kavarthapu, PhD, Staff Fellow
- Muruganath Kumar Raju, PhD, Postdoctoral Fellow
- Rajakumar Anbazhagan, PhD, Visiting Fellow
We investigate the molecular basis of peptide hormone control of gonadal function, with particular emphasis on the structure and regulation of the genes encoding the luteinizing hormone receptor (LHR) and prolactin (PRLR) receptor. We also investigate the regulatory mechanism(s) involved in the progression of spermatogenesis and the control of Leydig cell (LC) function. Our studies focus on the regulation of human LHR transcription (nuclear orphan receptors, epigenetics, DNA methylation, second messengers, repressors, corepressors, and coactivators), as well as on the multiple-promoter control of hPRLR gene transcription. We are elucidating the relevance of prolactin (PRL), estradiol and its receptor (liganded or un-liganded), epidermal growth factor (EGF), and the EGF receptors ERRBB1/EGFR and ERRB2/HER2 in the up-regulation of the PRLR, and their mechanistic commonalities for definition of PRL/PRLR–induced progression and metastasis of breast tumors, as well as their role in persistent invasiveness in certain states refractory to adjuvant endocrine therapies. We also investigate novel gonadotropin-regulated genes relevant to the progression of testicular gametogenesis, LC function, and other endocrine processes. We focus on the function and regulation of the gonadotropin-regulated testicular RNA helicase (GRTH/DDX25), an essential post-transcriptional regulator of spermatogenesis, which was discovered, cloned, and characterized in our laboratory. The various functions of GRTH/DDX25 provide a fertile ground for the development of a male contraceptive.
The luteinizing hormone receptor
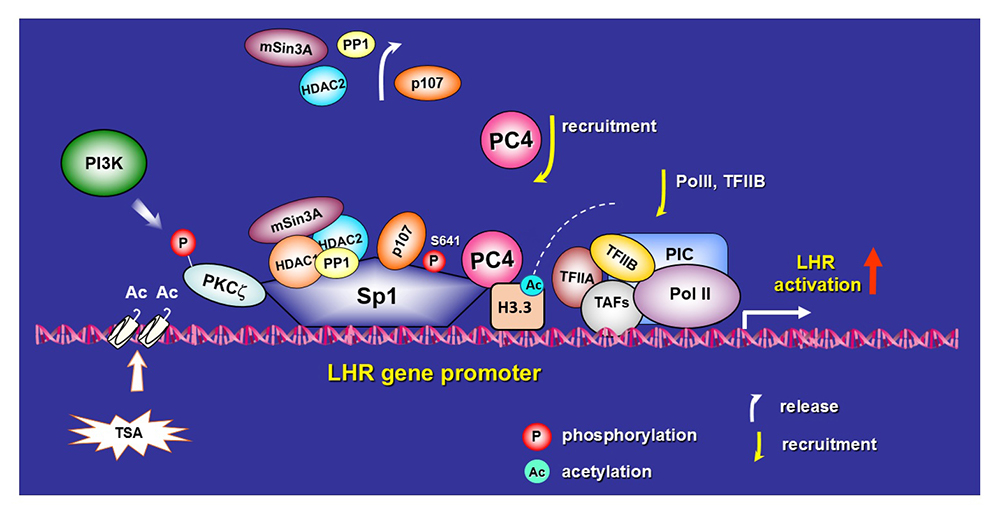
The luteinizing hormone receptor (LHR) is expressed primarily in the gonads, where it mediates LH signaling, which regulates ovarian and testicular function. The human LHR gene is transcriptionally controlled by diverse networks, in which coordination and interactions between regulatory effectors are essential for silencing/activation of LHR expression. The proximal Sp1 promoter site for transcription factor Sp1 recruits histone (H) deacetylases (HDAC) and the Sin3A corepressor complex, which contribute to the silencing of LHR transcription. Site-specific acetylation/methylation induced by trichostatin A (TSA), an inhibitor of histone deacetylase, causes phosphatase release, which serves as an on switch for Sp1 phosphorylation, which in turn causes p107 repressor release from Sp1, recruitment of Transcription Factor II B (TFIIB) and RNA polymerase II (Pol II), and transcriptional activation. Positive Cofactor 4 (PC4) is recruited by Sp1, acts as its coactivator, and has an essential role in the formation/assembly of the preinitiation complex (PIC) and TFIIB and Pol II recruitment in TSA–mediated LH transcription.
Our recent studies demonstrated an association between PC4 and acetylated histone H3 in TSA–induced LHR de-repression in MCF7 cells. Tandem mass spectrometry (MS/MS) studies revealed an association of PC4 with the H3.3 variant acetylated at several Lys residues (K9, K14, K18, K23, K27). We further confirmed the presence of these modifications by site-specific H3 antibodies in Western blots. ChiP/reChiP analysis showed an increased recruitment of complexes of PC4/acetylated H3 at these sites to the LHR promoter upon TSA stimulation. Further, immunoprecipitation (IP) studies of cells transfected with PC4–Flag-by-Flag antibody demonstrated interaction of PC4 with H3.3 induced by TSA, using H3.3–specific antibody, and the presence of the complex PC4-H3.3 at the LHR promoter was demonstrated by reChiP. Depletion of endogenous PC4 or H3.3 A/B by siRNA caused marked reduction of TSA–induced formation of the complex, its recruitment to the promoter and of transcriptional activation of the LHR gene. In recent studies, we found that this resulted from a lowered accessibility of the chromatin at the promoter region of the LHR, as indicated by the relative increases in DNAse protection in cells with H3.3 knock-down. Recent pull-down studies demonstrated association of H3/H3.3 with GST–PC4 in MCF7-extracts, whereas we found no direct association with histone H4. Similarly, we observed direct association with recombinant H3 and H3.3 proteins in a GST–PC4 pull-down assay, indicating direct association with H3 and H3.3 but not with H4. Given that addition of H3-H4 or H3.3-H4 tetramer revealed H3 and H4 or H3.3 and H4 protein bands, respectively, we conclude that PC4 associates with the tetramer via H3 or H3.3 in vitro. This is in contrast to findings in Flag-PC4–transfected cells, in which PC4 associates with endogenous histone protein but not with the tetramer, as evidenced by the sole presence of H3 and absence of H4 in IP with Flag antibody. This could indicate dissociation of the tetramer resulting from the association with the PC4-Sp1 complex. The recruitment of PC4 to Sp1 and formation of PC4–Sp1 complex was previously shown to be essential for LHR transcription. PC4 expression is not affected by TSA, therefore PC4 levels do not relate to changes in H3/H3.3–Ac expression. However, PC4–H3.3 interactions favor histone acetylation. Knock-down of endogenous PC4 in MC7 cells resulted in significant reduction of H3K–acetylated protein expression. TSA induced enrichment of acetylated H3K9 (H3K9–Ac) and H3.3K9–Ac at the promoter and consequently of LHR transcriptional activity.
Our studies have linked acetylation of H3 to PC4, given that its absence abolished H3 acetylation induced by TSA, despite of mayor increases in expression of both H3 and H3.3 protein induced by TSA. Acetylation of H3.3 leads to chromatin accessibility and gene transcription. Taken together, the findings indicate a critical role of PC4 association with acetylated H3.3 in TSA–induced Sp1–activated LHR transcription [Reference 1] (Figure 1).
Click image to view.
Figure 1. PC4 interaction with H3.3 in TSA–induced luteinizing hormone receptor (LHR) gene transcriptional activation: sequence of events leading to activation of LHR gene transcription
Activation of the LHR gene is achieved by the combined actions of chromatin changes, release of inhibitory factors (p107, HDAC1/2/mSin3A, and phosphatase PP1), phosphorylation of the transcription factor Sp1 at Ser641 by PI3K/PKC zeta, and recruitment of Positive Cofactor 4 (PC4). PC4 functions as a Sp1 coactivator and as a linker to bridge Sp1 to acetylated H3.3. These modifications lead to an increase in chromatin accessibility, recruitment of Transcription Factor II B (TFIIB) and RNA polymerase II (Pol II), and transcriptional activation during LHR gene derepression induced by TSA [Reference 1].
Gonadotropin-regulated testicular RNA helicase
Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) is a testis-specific member of the DEAD-box family of RNA helicases present in Leydig cells (LC) and meiotic germ cells and is essential for the completion of spermatogenesis. Males lacking GRTH are sterile owing to azoospermia resulting from failure of round spermatids to elongate. We demonstrated the enzyme's participation in the nuclear export/transport of specific mRNAs, the structural integrity of the Chromatoid Body storage/processing of relevant mRNAs, and their transit/association to the actively translating polyribosomes, where it may regulate translational initiation of genes. GRTH is regulated by LH through the androgen (A)/androgen receptor (AR) at the transcriptional level in LCs (direct), with impact on hCG–induced steroidogenesis, while not affecting basal circulating levels (of testosterone) in mice, and in germ cells (indirectly via AR in Sertoli cells), where its expression is both cell- and stage-specific. Transgenic (Tg) mouse models generated in our laboratory carrying GRTH 5′ flanking regions–GFP provided in vivo systems that permitted differential elucidation of regions in the GRTH gene that direct its expression (upstream) in germ cells (pachytene spermatocytes and round spermatids) and downstream in LCs, and its direct autocrine regulation by A/AR in LCs, and indirect paracrine regulation in germ cells. Functional GRTH binding sites for germ cell nuclear factor (GCNF, an orphan nuclear receptor-transcription factor that regulates GRTH transcription) ) and the helicase's regulation by A/AR were identified in the distal region of the gene. The studies provided evidence for actions of A on GCNF cell-specific regulation of GRTH expression, which operates selectively in round spermatids. Also, GRTH exerts negative autocrine regulation of GCNF. Our in vivo/in vitro models, linking A actions to germ cells through GCNF as an A–regulated trans-factor that controls transcription/expression of GRTH, provide a connection between A action and two relevant germ cell genes essential for the progress of spermatogenesis and establish their regulatory relationship.
Our early studies revealed that a missense mutation of R to H at amino acid 242 of GRTH found in 5.8% of patients with non-obstructive azoospermia, when expressed in COS1 cells, causes loss of the 61 KDa cytoplasmatic phospho-species with preservation of the nuclear 56 KDa non-phospho form. The finding provided an avenue to elucidate the function of phospho–GRTH in spermatogenesis. We generated a humanized mutant GRTH knock-in (KI) mouse. Recent studies revealed that mutant KI mice are sterile with marked reduction in the size of the testes, which lack sperm, and with arrest at step 8 of round spermatids and complete loss of the phospho–GRTH species but with preservation of the non-phospho form. The mouse model will permit us to discern the biological and biochemical impact of the phospho-species in GRTH function. In recent studies, we elucidated the GRTH phospho-site at a threonine structurally adjacent to the mutant site found in patients. Molecular modelling of the phospho-site, based on the crystal structure of DDX19, which shares 64% amino acid identity with the GRTH/DDX25 discovered in our lab (because we do not have the crystal structure of GRTH, we modeled the phospho-site based on the crystal structure of DDX19), elucidated the relevant amino acids that form the GRTH/PKA (protein kinase A) interface. Studies based on the abolition of the phospho-form provide the basis for drug design and virtual and throughput screening to find a reversible chemical inhibitor that coould be used as a male contraceptive.
The prolactin receptor
The human prolactin receptor (PRLR) mediates the diverse cellular actions of prolactin (PRL) and has an important role in the etiology and progression of breast cancer, tumoral growth, and chemo-resistance. Our studies have elucidated the relevance of PRL, estradiol (E2) and its liganded or unliganded receptor, epidermal growth factor (EGF), epidermal growth factor receptors (ERBB1/EGFR), and ERBB2/HER2 (human epidermal growth factor receptor 2) in the up-regulation of the PRLR gene transcription/expression and their mechanistic commonalities for definition of PRL/PRLR–induced progression and metastasis of breast tumors, which could explain persistence and invasiveness in certain refractory states to adjuvant endocrine therapies. We demonstrated that the specific CDK7 kinase inhibitor THZ1, which inhibits E2–induced phosphorylation of estrogen receptor a (ERa) at S118, abrogated E2–induced PRLR transcription/expression and E2–induced cell migration. THZ1 singly or in combination with other approaches targeting PRLR expression, function/signaling, could abate PRLR transcription/expression and prevent deleterious effects of PRLR, fueled by tumor PRL, in breast cancer [Reference 2].
Publications
- Zhao P, Kavarthapu R, Anbazhagan R, Liao M, Dufau ML. Interaction of positive coactivator 4 with histone 3.3 protein is essential for transcriptional activation of the luteinizing hormone receptor gene. BBA-Gene Regul Mech 2018;1861:971-981.
- Kavarthapu R, Dufau ML. Essential role of endogenous prolactin and cdk7 in estrogen-induced regulation of the prolactin receptor in breast cancer cells. Oncotarget 2017;8:27353-27363.
Collaborators
- James M. Pickel, PhD, Transgenic Core Facility, NIMH, Bethesda, MD
Contact
For more information, email dufau@helix.nih.gov or visit irp.nih.gov/pi/maria-dufau.


