Thyroid Hormone Regulation of Vertebrate Postembryonic Development

- Yun-Bo Shi, PhD, Head, Section on Molecular Morphogenesis
- Liezhen Fu, PhD, Staff Scientist
- Nga Luu, MS, Biologist
- Keisuke Nakajima, PhD, Visiting Professor
- Wonho Na, PhD, Visiting Fellow
- Yuki Shibata, PhD, Visiting Fellow
- Yuta Tanizaki, PhD, Visiting Fellow
- Lingyu Bao, MS, Graduate Student
- Shouhong Wang, BS, Graduate Student
- Lataijah C. Crawford, BS, Postbaccalaureate Fellow
- Robert Liu, BS, Postbaccalaureate Fellow
This laboratory investigates the molecular mechanisms of thyroid hormone (TH) function during postembryonic development. The main models are the metamorphoses of Xenopus laevis and X. tropicalis, two closely related species, which offer unique but complementary advantages. The control of the developmental process by TH offers a paradigm to study gene function in postembryonic organ development. During metamorphosis, various organs undergo vastly different changes. Some, like the tail, undergo complete resorption, while others, such as the limb, are developed de novo. The majority of the larval organs persist through metamorphosis but are dramatically remodeled to function in a frog. For example, tadpole intestine is a simple tubular structure consisting primarily of a single layer of larval epithelial cells. During metamorphosis, through specific larval epithelial cell death, it is transformed into an organ with a many-folded adult epithelium surrounded by elaborate connective tissue and muscles and by de novo development of the adult epithelial stem cells followed by their proliferation and differentiation. The wealth of knowledge from past research and the ability to manipulate amphibian metamorphosis, both in vivo by using genetic approaches or hormone treatment of whole animals, and in vitro in organ cultures, offer an excellent opportunity to: (1) study the developmental function of TH receptors (TRs) and their underlying mechanisms in vivo; and (2) identify and functionally characterize genes that are critical for organogenesis, particularly for the formation of the adult organ-specific stem cells, during postembryonic development in vertebrates. A major recent focus has been to make use of the TALEN and CRISPR/Cas9 technologies to knock down or knock out endogenous genes for functional analyses.
Investigation of the function of endogenous TR genes using knockout animals
By using the TALEN technology, we had previously generated X. tropicalis animals lacking any functional TRα and observed that TRα knockout animals are surprisingly able to complete metamorphosis at a similar age as the wild-typing siblings [Reference 1]. However, careful analyses during development revealed many roles of TRα during Xenopus development, including preventing precocious initiation of metamorphosis when TH is absent, regulating the rate of metamorphic progression, and coordinating temporal regulation of metamorphosis in different tissues/organs [Reference 1]. We have now also generated TRβ heterozygous knockout animals and begun to analyze the role of TRβ in Xenopus development. In addition, global RNA sequencing (RNA-seq) and chromatin-immunoprecipitation (ChIP) sequencing (ChIP-seq) are being carried out on wild-type and TR–knockout animal tissues to determine the molecular pathways regulated by distinct TRs in different tissues during metamorphosis.
Click image to view.
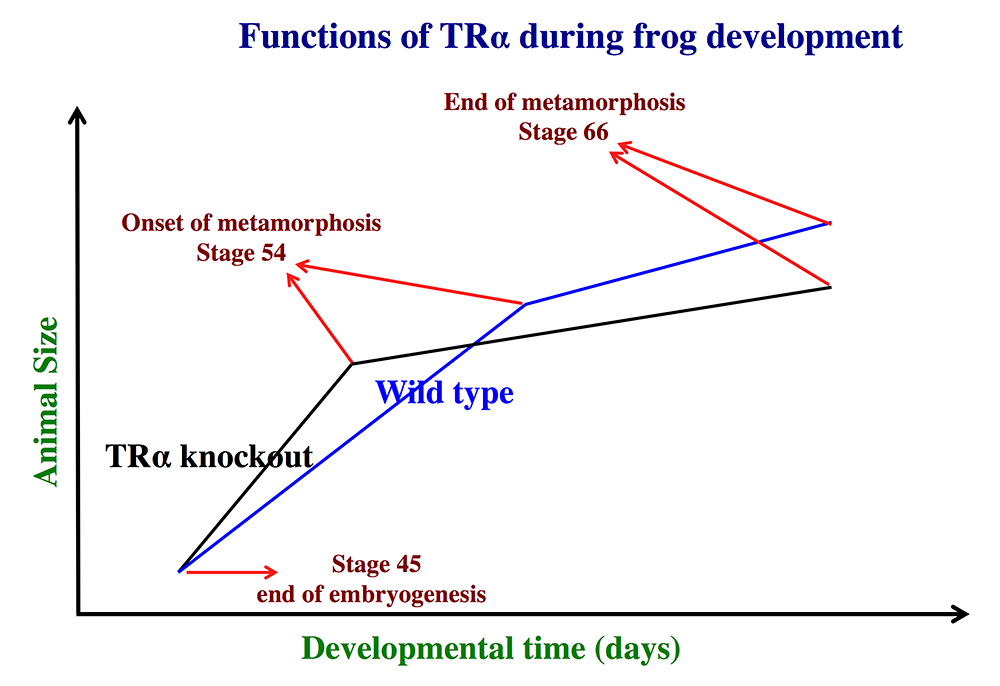
Figure 1. Schematics showing the effects of TRα (thyroid hormone α) knockout on Xenopus tropicalis development
TRα knockout has little effect on embryogenesis, and resulting tadpoles are normal by feeding stage (stage 45). Once feeding begins, the animals grow at different rates, with the knockouts growing faster; they are thus larger than wild-type siblings at the same age (in days) (comparing the vertical axis values of the lines for the knockout and wild-type animals at any given position along the horizontal axis between stages 45 and 54). The knockout animals also develop faster, reaching developmentally more advanced stages than wild-type siblings at the same age (in days). Thus, the knockout animals reach stage 54, the onset of metamorphosis, at a younger age (see the horizontal axis locations for the upper end of the lines). Interestingly, when the animals are compared at stage 54, the wild-type are larger than the knockout siblings, even though the latter grow faster. This is because the wild-type animals take longer to reach metamorphosis (stage 54). The extra growth time needed to reach stage 54 enables the wild-types to catch up and surpass the knockouts in size. After the initiation of metamorphosis at stage 54, the knockout tadpoles metamorphose more slowly than the wild-type ones, enabling the latter to catch up in development, with both groups finishing metamorphosis at around the same age. The knockout animals initiate metamorphosis at a smaller size and also end up with a smaller size at the end of metamorphosis than do the wild-type siblings. Thus, in premetamorphic tadpoles prior to stage 54, unliganded TRα (due to the lack of thyroid hormone) functions to control metamorphic timing, whereas, when thyroid hormone becomes available during metamorphosis, TRα helps increase the rate of metamorphosis.
Genome-wide identification of thyroid hormone receptor targets in the remodeling intestine during X. tropicalis metamorphosis
We have been studying how TH regulates adult stem cell development by using TH–regulated intestinal metamorphosis as a model system. A key step is to identify and functionally characterize direct TH target genes during this process. Prior to the release of a well annotated Xenopus genome, we generated a set of genomic microarray chips covering about 8,000 bp flanking each predicted transcription start site in X. tropicalis genome. We used the chips for chromatin immunoprecipitation assays (ChIP-on-chip) on the intestine of premetamorphic tadpoles treated with or without TH for genome-wide identification of TR binding sites. We have now completed and published this study [Reference 2]. Our work led to the identification of 278 candidate direct TR target genes. Further, we provided evidence that the genes are regulated by TH and likely involved in the TH–induced formation of adult intestinal stem cells during metamorphosis [Reference 2].
The histone methyltransferase DOT1L is a coactivator for the thyroid hormone receptor during Xenopus development.
Click image to view.
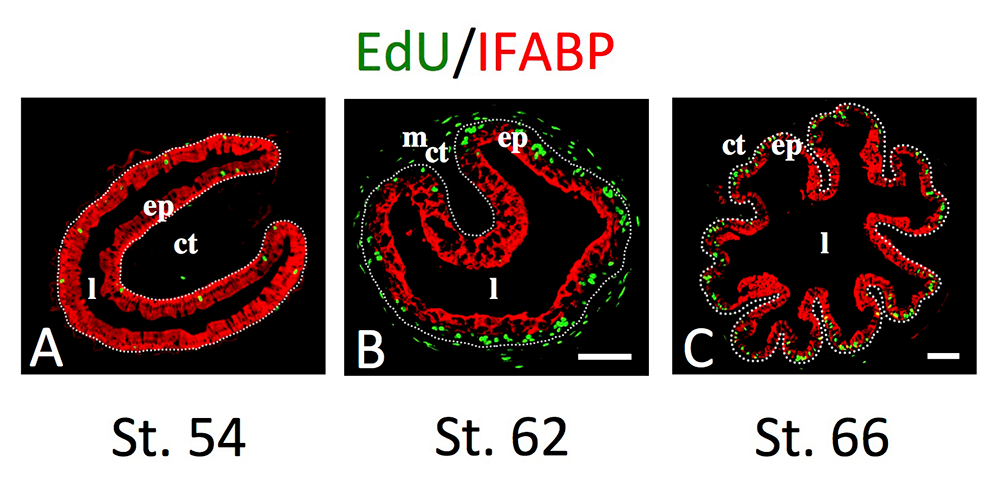
Figure 2. Intestinal metamorphosis involves the formation of clusters of proliferating, undifferentiated epithelial cells at the climax.
Tadpoles at premetamorphic stage 54 (A), climax (B, stage 62), and end of metamorphosis (C, stage 66) were injected with 5-ethynyl-2′-deoxyuridine (EdU) one hour before being sacrificed. Cross-sections of the intestine from the resulting tadpoles were double-stained by EdU labeling of newly synthesized DNA and by immunohistochemistry of IFABP (intestinal fatty acid–binding protein), a marker for differentiated epithelial cells. The dotted lines depict the epithelium-mesenchyme boundary. Note that there are few EdU–labeled proliferating cells in the epithelium and that they express IFABP at premetamorphosis (A) and increase in the form of clustered cells (proliferating adult stem cells) that lack IFABP at the climax of metamorphosis (B). At the end of metamorphosis, EdU–labeled proliferating cells are localized mainly in the troughs of the epithelial folds, where IFABP expression is low (C). ep: epithelium; ct: connective tissue; m: muscles; l: lumen.
Histone modifications are associated with transcriptional regulation by diverse transcription factors. Genome-wide correlation studies revealed that histone activation marks and repression marks are associated with, respectively, activated and repressed gene expression. One of the histone activation marks is histone H3 K79 methylation, which is carried out by only a single methyltransferase, Dot1L. Interestingly, the ChIP-on-chip analysis mentioned above revealed that Dot1L is regulated by TH at the transcriptional level, and our previous studies showed that H3K79 methylation levels are induced at TH target genes during natural and TH–induced metamorphosis. The findings suggest that TH induces Dot1L expression and that Dot1L, in turn, functions as a TR coactivator to promote vertebrate development. In co-transfection studies or in the reconstituted frog oocyte in vivo transcription system, we showed that overexpression of Dot1L enhances gene activation by TR in the presence of TH. Making use of the ability to carry out transgenesis in X. laevis and gene knockdown in X. tropicalis, we further demonstrated that endogenous Dot1L is critical for TH–induced activation of endogenous TR target genes, while transgenic Dot1L enhances endogenous TR function in premetamorphic tadpoles in the presence of TH. Our studies thus provide, for the first time, complementary gain- and loss-of-functional in vivo evidence for a cofactor, Dot1L, in gene activation by TR during vertebrate development [Reference 3].
EVI and MDS/EVI are required for adult intestinal stem cell formation during postembryonic vertebrate development.
As indicated above, we have been using intestinal metamorphosis as a model to study the development of adult organ-specific stem cells in vertebrates. During metamorphosis, the larval epithelial cells in the tadpole intestine undergo apoptosis in response to the rising concentration of TH. Concurrently, a small fraction of the epithelial cells, by a yet unknown mechanism, undergo TH–dependent dedifferentiation to become adult stem cells [Reference 4]. We had previously carried out a microarray analysis to identify genes that are regulated by TH in the intestinal epithelium. Among the genes discovered are the genes ectopic viral integration site 1 (EVI) and its variant, myelodysplastic syndrome 1 (MDS/EVI). They both encode zinc-finger proteins, which have been recognized as important oncogenes in various types of cancer. In contrast to the established role of EVI and MDS/EVI in cancer development, their potential function during vertebrate postembryonic development, especially in organ-specific adult stem cells, is unclear. We showed that high levels of EVI and MDS/EVI are expressed in the intestine at the climax of metamorphosis and induced by TH. By using the TALEN gene-editing technology, we knocked out both EVI and MDS/EVI and showed that EVI and MDS/EVI are not essential for embryogenesis and premetamorphosis in X. tropicalis [Reference 5]. On the other hand, knocking out EVI and MDS/EVI causes severe retardation in the growth and development of the tadpoles during metamorphosis and leads to tadpole lethality at the climax of metamorphosis. Furthermore, the homozygous knockout animals exhibit reduced adult intestinal epithelial stem cell proliferation at the end of metamorphosis (for the few that survive through metamorphosis) or during TH–induced metamorphosis. The findings reveal a novel role of EVI and/or MDS/EVI in regulating the formation and/or proliferation of adult intestinal stem cells during post-embryonic development in vertebrates [Reference 5].
Additional Funding
- Japan Society for the Promotion of Science (JSPS) fellowships for Drs. Yuta Tanizaki and Yuki Shibata
- Lataijah C. Crawford was an NICHD Developing Talent Scholar.
- An award from the China Scholarship Council (CSC) to Shouhong Wang
- Keisuke Nakajima was supported by Hiroshima University, Japan
Publications
- Wen L, Shibata Y, Su D, Fu L, Luu N, Shi Y.B. Thyroid hormone receptor a controls developmental timing and regulates the rate and coordination of tissue specific metamorphosis in Xenopus tropicalis. Endocrinology 2017;158:1985-1998.
- Fu L, Das D, Matsuura K, Fujimoto K, Heimeier RA, Shi Y-B. Genome-wide identification of thyroid hormone receptor targets in the remodeling intestine during Xenopus tropicalis metamorphosis. Sci Rep 2017;7,6414:1-11.
- Wen L, Fu L, Shi Y-B. Histone methyltransferase Dot1L is a coactivator for thyroid hormone receptor during Xenopus development. FASEB J 2017;31:4821-4831.
- Okada M, Shi Y-B. Cell proliferation analysis during Xenopus metamorphosis. Cold Spring Harb Protoc: Xenopus 2017;696-701.
- Okada M, Shi Y-B. EVI and MDS/EVI are required for adult intestinal stem cell formation during postembryonic vertebrate development. FASEB J 2018;32:431–439.
Collaborators
- Sheue-Yann Cheng, PhD, Laboratory of Molecular Biology, NCI, Bethesda, MD
- Jianping Jiang, PhD, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China
- Peter Taylor, PhD, University of Dundee, Dundee, UK
- Bingyin Shi, MD, Xi’an Jiaotong University School of Medicine, Xi'an, China
- Guihong Sun, PhD, Wuhan University School of Medicine, Wuhan, China
Contact
For more information, email shi@helix.nih.gov or visit http://smm.nichd.nih.gov.


