Regulation and Functional Relevance of Retrograde Axonal Transport in Axons

- Catherine Drerup, PhD, Head, Unit on Neuronal Cell Biology
- Katherine Pinter, BSc, Technician
- Amrita Mandal, PhD, Visiting Fellow
- Dane Kawano, BSc, Postbaccalaureate Fellow
- Wesley Schnapp, BSc, Postbaccalaureate Fellow
- Serena Wisner, BS, Postbaccalaureate Fellow
Cytoplasmic dynein is the single motor responsible for microtubule minus-end (cell body–directed) axonal transport. The importance of this retrograde motor to neural health is apparent: mutations in the dynein-dynactin complex are causal in subtypes of neurological disease. Additionally, the abnormal localization of dynein-dependent cargos is associated with disease states. Though dynein function appears essential for neural health, the mechanisms that govern precise cargo movement by this motor and how that impacts neural circuit structure and function are almost completely unknown. One dynein cargo of critical importance to axonal physiology is the mitochondrion. Axons depend on the proper localization of mitochondria for sufficient ATP synthesis and calcium buffering, as well as for lesser known functions such as production or metabolites, synthesis of signaling molecules, and iron homeostasis. Abnormalities in mitochondrial localization are correlated with neurological disease; however, whether there is a causal relationship between organelle movement and disease has been difficult to determine. Our work on mitochondrial transport and function in relation to neural circuit activity aims at an understanding of the regulation of retrograde axonal transport and how it impacts the nervous system.
Using novel tools that we developed, namely, forward and reverse genetics, advanced imaging of intracellular phenomena in vivo, and analyses of neural circuit function, we interrogate the molecular regulation of cargo-specific retrograde transport in axons and determine the role that movement of each of these cargos plays in the formation and maintenance of functional neural circuits.
Regulation and functional significance of retrograde axonal transport
Mitochondrial retrograde transport mechanisms and function
Mitochondrial transport is necessary to properly position the organelle in axons. Correct mitochondrial localization in axons is critical for energy production near sites of high metabolic demand, for calcium homeostasis to regulate neuronal activity, and to regulate axonal branching in certain contexts. Various model systems have revealed several mediators of mitochondrial movement. Anterograde mitochondrial transport requires the Kinesin-1 molecular motor in association with several other proteins, including Miro and Milton. Interestingly, loss of Miro or Milton, the two best characterized adaptors for mitochondrial transport, eliminates all mitochondrial movement. Therefore, how this organelle is selectively transported in the retrograde direction is still unclear.
Click image to view.
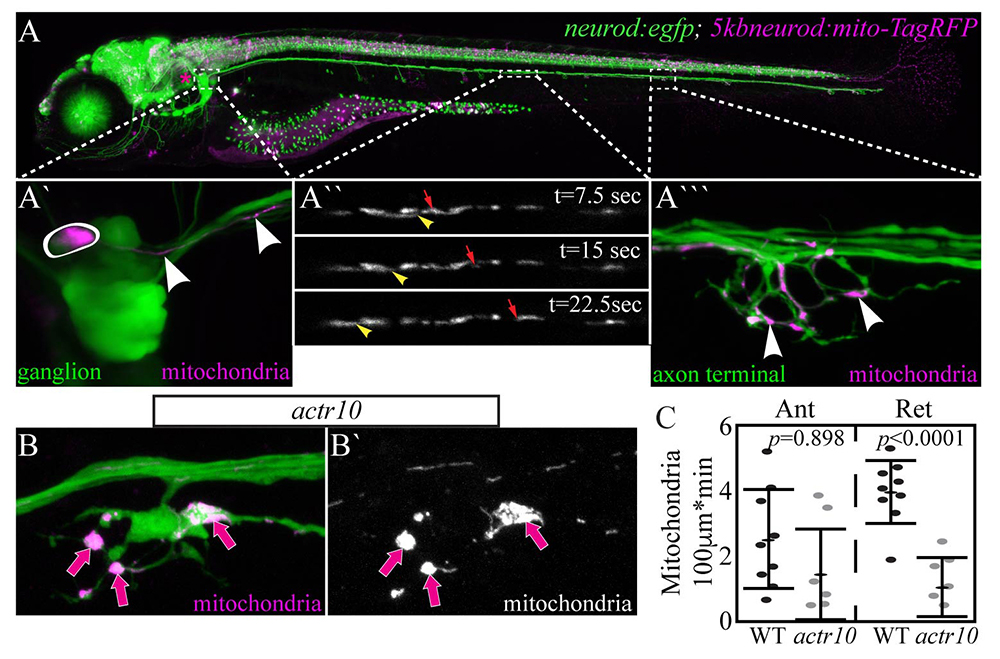
Figure 1. Mitochondria accumulate in actr10–mutant axon terminals due to failed retrograde transport.
A. 5–dpf (days post-fertilization) TgBAC(neurod:egfp)n11 transgenic zebrafish larva expressing GFP in all neurons. Mitochondrially localized TagRFP mosaically expressed.
A`. Posterior lateral line (pLL) ganglion with one neuron expressing TagRFP in mitochondria (circled). Arrowheads point to mitochondria in the single labeled axon.
A``. Mitochondrial transport in a single pLL axon. Arrowhead: retrograde. Arrow: antereograde.
A```. Arrowheads point to mitochondria in the axon terminal of this system.
B. Mitochondrial accumulation in actr10–mutant axon terminals (arrows).
C. Retrograde mitochondrial transport is disrupted in this mutant strain.
In a forward genetic screen, we identified a novel zebrafish mutant strain with selective loss of retrograde mitochondrial transport. The causative mutation in this line causes loss of Actr10, a protein known to associate with the dynein motor. In actr10 mutants, anterograde mitochondrial transport is intact but retrograde mitochondrial transport frequency is dramatically reduced, leading to accumulation of mitochondria in actr10 mutant axon terminals (Figure 1). Using mitochondrial fractionation, we demonstrated that the loss of retrograde transport frequency is attributable to loss of mitochondria-dynein interaction in the absence of Actr10. We are currently using the actr10 mutant as a tool to determine the impact of mitochondrial retrograde transport disruption on mitochondrial health and function. Additionally, we are working to address the normal rate and function of mitochondrial turnover in axons. For this, we made a stable transgenic zebrafish line expressing the photo-convertible protein mEos in the inner mitochondrial membrane space. Somewhat surprisingly, just three hours after photo-conversion, the old (converted) mitochondria had evacuated the axon terminal and new (unconverted) had taken their place (Figure 2). This is in contrast to the predictions based on shorter-term imaging done on the order of minutes in cultured neurons. The findings demonstrate the power of an entirely in vivo system for observing cellular phenomena on a time-scale relevant to the organism. Finally, in collaboration with the Kindt lab, we are investigating the impact of mitochondrial accumulation on the health and function of the axon. Together, the work will define the molecular mechanisms of retrograde mitochondrial transport and provide insights into how loss of this specific cellular activity impacts the organelle, the cell, and the neural circuit in vivo.
Click image to view.
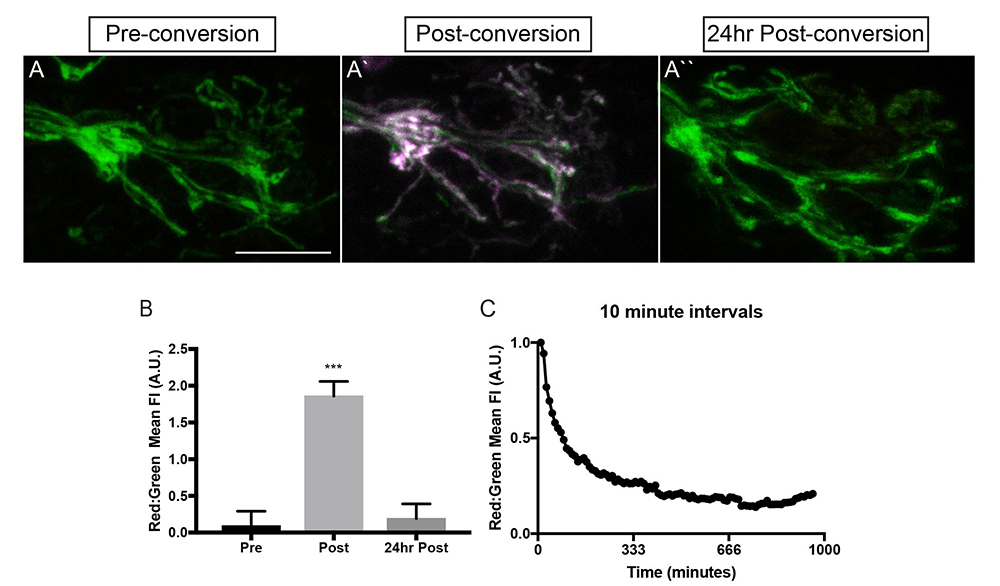
Figure 2. Mitochondria turn over in axon terminals within hours.
A. Photoconversion of mEos in the inner mitochondrial membrane space labels axon terminal mitochondria (magenta in A`). Twenty-four hours after conversion, no converted (old) mitochondria remain.
B. Quantification of old (red) to new (green) mitochondria before (pre), just after (post), and 24hr after (24hr post) conversion.
C. Time-lapse imaging revealed that mitochondrial turnover occurs about 3hrs after conversion in 4–5 dpf (days post-fertilization) larvae.
Microtubules, dynein stability, and NudC
Our forward-genetic screen yielded several other mutant lines, including one with a loss-of-function mutation in the dynein-associated protein NudC. In mammalian systems, loss of NudC results in failed mitosis and abnormal neuronal migration. The function of NudC in these phenomena is still under investigation. During cell division, loss of NudC results in abnormal microtubule orientation and failure of kinetochore complex formation. Work in migrating neurons indicated that loss of NudC impacts the stability of the dynein motor protein complex. Whether NudC has similar or disparate functions during different stages of development in neurons is thus still highly debated. The NudC mutant identified in our screen will allow us to address the role of NudC in mature axons in vivo.
NudC mutants do not phenocopy dynein or dynactin loss-of-function mutants, with only axon branchpoint swellings in distal axons and no signs of either axonal or retinal degeneration. In collaboration with the Petralia lab, we used transmission electron microscopy to reveal that loss of NudC results in multilamellar body formation in the swellings (Figure 3). In vivo analyses of axonal transport of autophagosome- and endosome-related cargos revealed that most show disruption of their axonal transport; however, the disruptions are not consistent, leading us to ask whether the structure or arrangement of the microtubule cytoskeleton is effected in this line. Currently, we are using pharmacology, live imaging of microtubule dynamics, and expansion microscopy to gain insight into the structure, stability, and dynamics of the microtubule cytoskeleton in this line to understand the function of NudC in a mature axon. The culmination of this work will allow us to determine whether NudC serves a conserved function in developing neurons, which, when disrupted, leads to varying phenotypes based on developmental state.
Click image to view.
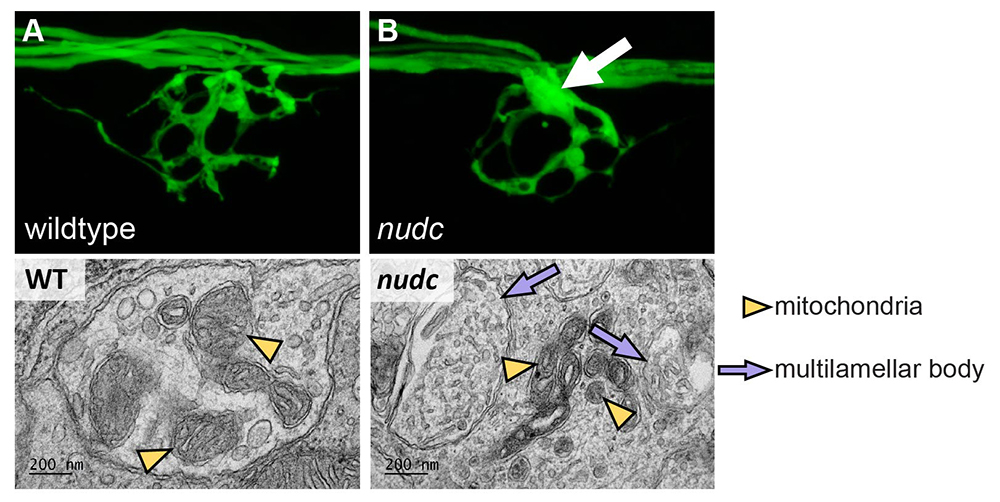
Figure 3. nudc mutants show multilamellar body formation in axon terminal branchpoint swellings.
A. Wild-type axon terminal of an afferent axon terminal in the posterior lateral line system of a zebrafish larva at 4 dpf (days post-fertilization).
B. nudc axon terminal branchpoints show swellings by this time-point (arrow).
Bottom. Axon branchpoint swellings in nudc mutants show multilamellar body formation.
Screening for novel regulators of cargo-specific retrograde axonal transport
Several pieces of evidence substantiate the importance of retrograde axonal transport for axon health and function. First, mutations in dynein and dynein-associated proteins are correlated with neurological disease. Second, retrograde transport of signaling endosomes is essential for the extension and maintenance of long axons. Third, abnormal localization of various cargoes, including mitochondria, correlates with neuronal disease. Despite the importance of the process, little is known about how various cargoes attach to and are then transported by the retrograde motor protein complex. We are using forward and reverse genetics in zebrafish to identify mediators of dynein-specific retrograde transport in axons.
Using a double transgenic line in a three-generation, forward genetic screen, we are identifying recessive mutant strains with axon abnormalities characteristic of disruptions in retrograde axonal transport. Our previous work and that of others showed that these phenotypes include axon terminal swellings such as those observed in actr10 mutants (Figure 3). After identifying the strain, we use RNA–sequencing approaches to identify the causal mutation. To date, we have identified three lines, which all have mutations in dynein-associated proteins. At present, we are using the immuno-labeling approaches and in vivo imaging techniques that we previously developed to determine whether the strains have deficits in the retrograde transport of specific cargoes. We can use the mutant strains to first identify the proteins involved in the retrograde transport of particular cargoes and then as tools to determine how the specific disruptions affect the function of the axon.
Publications
- Mandal A, Pinter K, Drerup CM. Analyzing neuronal mitochondria in vivo using fluorescent reporters in zebrafish. Front Cell Dev Biol 2018;6:144.
- Drerup CM, Herbert AL, Monk KR, Nechiporuk A. Regulation of mitochondria-dynactin interaction and mitochondrial retrograde transport in axons. eLife 2017;6:22234.
- Drerup CM, Nechiporuk A. In vivo analysis of axonal transport in zebrafish. Methods Cell Biol 2016;131:311-329.
- Herbert AL, Fu MM, Drerup CM, Gray RS, Harty BL, Ackerman SD, O’Reilly-Pol T, Johnson SL, Nechiporuk AV, Barres BA, Monk KR. Dynein/dynactin is necessary for anterograde transport of Mbp mRNA in oligodendrocytes and for myelination in vivo. Proc Natl Acad Sci USA 2017;114:E9153-E9162.
Collaborators
- Tory Herman, PhD, University of Oregon, Eugene, OR
- Katie Kindt, PhD, Section on Sensory Cell Development and Function, NIDCD, Bethesda, MD
- Ronald Petralia, PhD, Advanced Imaging Core, NIDCD, Bethesda, MD
- Michael Ward, MD, PhD, Inherited Neurodegenerative Diseases Unit, NINDS, Bethesda, MD
Contact
For more information, email katie.drerup@nih.gov or visit http://dreruplab.nichd.nih.gov.


