Mechanisms of Disease in Preterm Labor and Complications of Prematurity; Prenatal Diagnosis of Congenital Anomalies

- Roberto Romero, MD, DMedSci, Chief, Perinatology Research Branch
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide. The cost of prematurity in the U.S. alone is estimated to be $26 billion per year. An important goal is to understand the mechanisms of disease responsible for spontaneous preterm birth and fetal injury and to develop methods for the prediction and prevention of preterm birth.
The Perinatology Research Branch (PRB) has proposed that preterm parturition is a syndrome caused by multiple pathologic processes, i.e., that preterm labor is one syndrome but has many causes. The emphasis of our Branch is to study intra-amniotic infection and inflammation, vascular disorders, maternal anti-fetal rejection (chronic inflammatory lesions of the placenta), cervical disease, and a decline in progesterone action. Previously, we reported that intra-amniotic inflammation, which affects at least one of every three preterm neonates, is characterized by the activation of amniotic-fluid neutrophils, cells that represent the first line of defense against infection. Using DNA fingerprinting, we determined that amniotic-fluid neutrophils are of fetal origin in cases of preterm labor, maternal origin in cases of clinical chorioamnionitis at term, and mixed origin in patients who have inflammatory processes near term. Moreover, in a series of studies, we were able to demonstrate that neutrophils produce antimicrobial peptides and exhibit the formation of extracellular traps, whereby they immobilize and kill bacteria.
The Branch also studies other obstetrical syndromes that account for the high rate of infant mortality in the United States, including clinical chorioamnionitis, which is the most common infection-related diagnosis in delivery units around the world, as well as meconium aspiration syndrome and amniotic fluid embolism.
Congenital anomalies continue to be a leading cause of perinatal mortality in the U. S. Imaging, a powerful tool for scientific discovery, has changed the practice of obstetrics and maternal-fetal medicine. Imaging with ultrasound allows the definition of fetal anatomy, biometry, growth, and the study of physiologic parameters, such as cardiac function, fetal sleep, and breathing. We invented a new method for the examination of the fetal heart, called fetal intelligent navigation echocardiography (FINE). This year, we reported a further major breakthrough: Color Doppler FINE. Color-flow mapping is essential for adequate examination of the fetal heart in those suspected of having congenital anomalies. We demonstrated how Color Doppler FINE can be used to improve the diagnosis of congenital anomalies. The technology has been licensed and is now commercially available to sonographers worldwide. This year, the sensitivity and specificity of FINE in fetuses with normal hearts and congenital heart disease in the second and third trimesters was reported for the first time.
Although ultrasound is the standard imaging modality in pregnancy, magnetic resonance imaging (MRI) has also been used to characterize fetal anatomy when ultrasound cannot provide definitive diagnostic answers. MRI provides unique information about fetal physiologic parameters (i.e., perfusion, oxygenation, and biochemistry) that are outside the domain of ultrasound. Moreover, MRI can be used to characterize the ontogeny of functional neuro-connectivity, as well as the potential relationship between insults that could alter fetal neuro-development. Given that preterm birth is a leading cause of neuro-developmental disorders, we used noninvasive methods to interrogate neuro-connectivity. In previous work, we reported a study showing that fetuses subsequently born preterm have a disorder of neuro-connectivity not seen in fetuses of the same gestational age subsequently born at term. Neuro-connectivity was reduced in the left hemisphere, close to the pre-language region, providing the first evidence that a disorder of functional connectivity is present in the fetus before birth.
Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations [Reference 1]
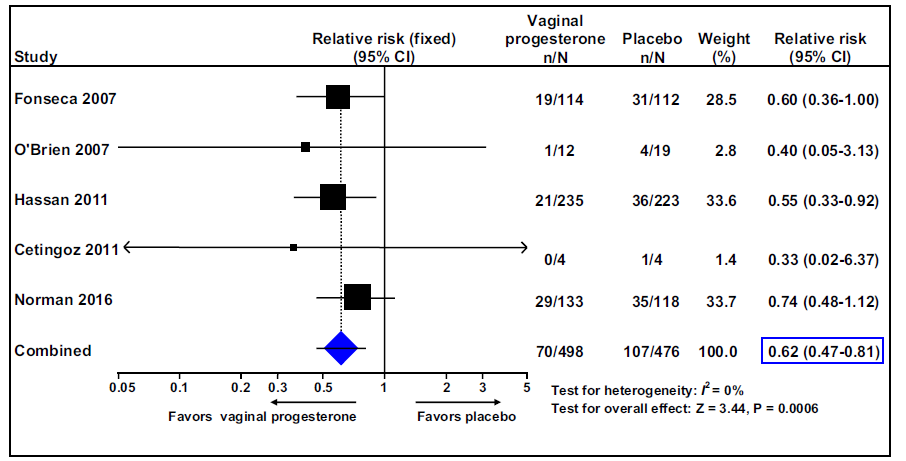
We conducted a meta-analysis of individual patient data to evaluate the efficacy of vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a sonographically detected short cervix in the midtrimester, including the data of the OPPTIMUM study. Trials comparing vaginal progesterone vs. placebo/no treatment in women with a singleton gestation and a midtrimester sonographic cervical length of 25 mm were included in this study. The primary outcome was preterm birth at less than (<) 33 weeks of gestation. Secondary outcomes included adverse perinatal outcomes and neuro-developmental and health outcomes at two years of age. Data were available from 974 women (498 allocated to vaginal progesterone, 476 allocated to placebo) with a cervical length of 25 mm participating in five high-quality trials.
Vaginal progesterone was associated with a significant reduction in the risk of preterm birth at < 33 weeks of gestation (Figure 1). Moreover, vaginal progesterone significantly reduced the risk of preterm birth at < 36, < 35, < 34, < 32, < 30, or <28 weeks of gestation; spontaneous preterm birth at <33 and <34 weeks of gestation; respiratory distress syndrome; composite neonatal morbidity and mortality; birthweight of < 1500 and < 2500 g; and admission to the neonatal intensive care unit. Maternal adverse events, congenital anomalies, and adverse neuro-developmental and health outcomes at two years of age did not differ between groups.
In summary, vaginal progesterone reduces the risk of preterm birth and improves perinatal outcomes in singleton gestations with a midtrimester sonographic short cervix, without any demonstrable harmful effects on childhood neuro-development.
Click image to view.
Figure 1.
The effect of vaginal progesterone on preterm birth at less than 33 weeks of gestation
Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation [Reference 2].
An indirect-comparison meta-analysis of individual patient data compared the efficacy of vaginal progesterone and cerclage in preventing preterm birth and adverse perinatal outcomes in women with a singleton gestation, previous spontaneous preterm birth, and a midtrimester sonographic short cervix. Both vaginal progesterone and cerclage, compared with placebo or no cerclage, significantly reduced the risk of preterm birth <35 and <32 weeks of gestation and composite perinatal morbidity/mortality. Adjusted indirect comparison meta-analyses did not show statistically significant differences between vaginal progesterone and cerclage in the reduction of preterm birth or adverse perinatal outcomes. We conclude that vaginal progesterone and cerclage are equally effective for preventing preterm birth and improving perinatal outcomes in women with a singleton gestation, previous spontaneous preterm birth, and a midtrimester sonographic short cervix.
Alloreactive fetal T cells promote uterine contractility in preterm labor [Reference 3].
Pregnancy is considered as a successful form of immune tolerance in which the mother accepts the semi-allogeneic fetus. Given that a breakdown of such tolerance is considered a mechanism for disease, several studies have focused on the mechanisms whereby the maternal immune system rejects the fetus. However, the fetal immune system has been somewhat disregarded. We hypothesized that the fetal immune system must also tolerate the mother and that a breakdown of such tolerance may likewise lead to pregnancy complications. Therefore, we investigated whether maternal antigen-specific activated fetal T cells contribute to the pathogenesis of spontaneous preterm labor.
We first demonstrated that antigen-presenting cells are activated in the umbilical cord blood of preterm neonates, which was accompanied by an increased proportion of pro-inflammatory memory CD4+ T cells together with a corresponding decline in naive CD4+ T cells, thus establishing that preterm neonates possess a distinct population of activated pro-inflammatory memory CD4+ T cells. Maternal cells can be present in the fetal circulation during pregnancy, where they promote the generation of fetal regulatory T cells in order to suppress fetal immune responses. However, the number of maternal cells present in the fetal circulation can increase in women with pregnancy complications. We therefore determined whether changes in these maternal cells in the cord blood are associated with preterm labor. We found that maternal cells were increased in the circulation of preterm neonates. CD4+ and CD8+ T cells from preterm neonates underwent more proliferation when presented with maternal antigens than those from term neonates; moreover, the response was specific to the mother, given that proliferation was not observed in response to antigens from third-party donors. Furthermore, T cells from preterm neonates expressed increased pro-inflammatory signals in response to stimulation with maternal antigens. Using an established model of myometrial contractility, we showed that unstimulated CD4+ and CD8+ T cells from preterm neonates caused increased contractility, suggesting that T cells from preterm neonates contribute to the onset of labor (Figure 2).
Click image to view.
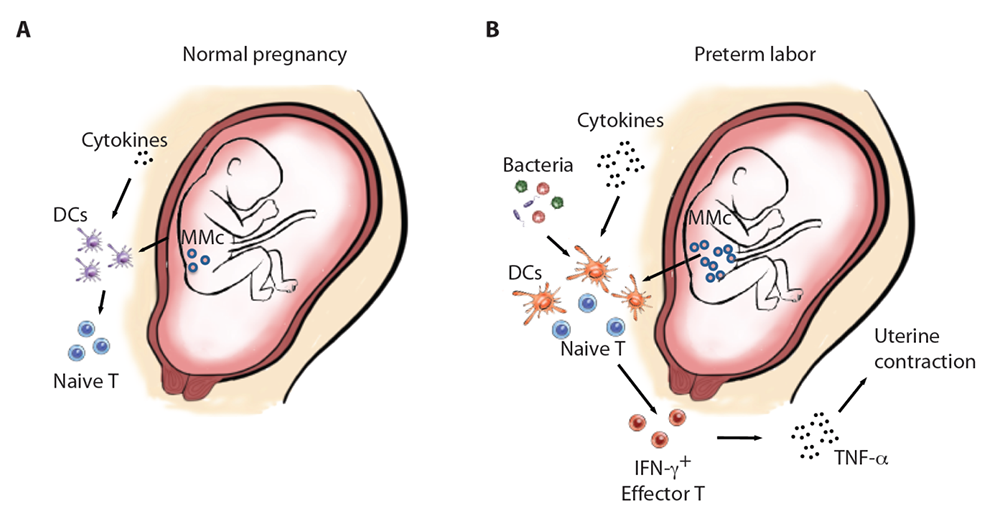
Figure 2. Schematic comparison of the fetal immune system between term and preterm labor
A. At baseline in term pregnancy, fetal DCs (dendritic cells) express low levels of co-stimulatory molecules, few maternal cells circulate in the fetal blood stream (maternal microchimerism, MMc), inflammatory cytokines in the fetal plasma are low, and the majority of fetal T cells are naive.
B. In the context of preterm labor, the MMc is elevated, and there is a possible presence of bacteria owing to PPROM, which could both serve as sources of antigens for DCs. Fetal DCs express higher levels of co-stimulatory molecules and present these antigens to naive T cells that subsequently will differentiate into effector cells with a Th1 phenotype and producing IFN-γ. Release of inflammatory cytokines such as TNF-α by activated fetal T cells results in uterine contraction.
Using a mouse model of in utero adoptive transfer, we also established that the presence of activated T cells in mouse fetuses in mid-pregnancy can result in pregnancy loss. Collectively, the findings demonstrate that fetal anti-maternal rejection is a novel mechanism for spontaneous preterm labor and are the first demonstration that the fetal immune system can reject the mother, leading to pregnancy termination.
A new customized fetal growth standard for African American women [Reference 4].
Current nomograms for the assessment of fetal growth in African American women were derived either from neonatal (rather than fetal) biometry data or were not customized for maternal ethnicity, weight, height, parity, and fetal sex. The goal of this study was to: (1) develop a new customized fetal growth standard for African American mothers; and (2) compare such a standard with three existing standards for the classification of fetuses as small (SGA) or large (LGA) for gestational age. A retrospective cohort study included 4183 women (4001 African American and 182 Caucasian) from the Detroit metropolitan area who underwent ultrasound examinations between 14 and 40 weeks of gestation (the median number of scans per pregnancy was 5, interquartile range 3–7) and for whom relevant covariate data were available. We used longitudinal quantile regression to build models defining the "normal" estimated fetal weight (EFW) centiles for gestational age in African American women, adjusted for maternal height, weight, parity and fetal sex, and excluding pathologic factors with a significant effect on fetal weight (Figure 3). We compared the resulting PRB/NICHD growth standard with three other existing standards: the customized gestation-related optimal weight (GROW) standard; the NICHD African American standard; and the multinational World Health Organization (WHO) standard, which is used to screen fetuses for SGA (<10th centile) or LGA (>90th centile) based on the last available ultrasound examination for each pregnancy.
Click image to view.
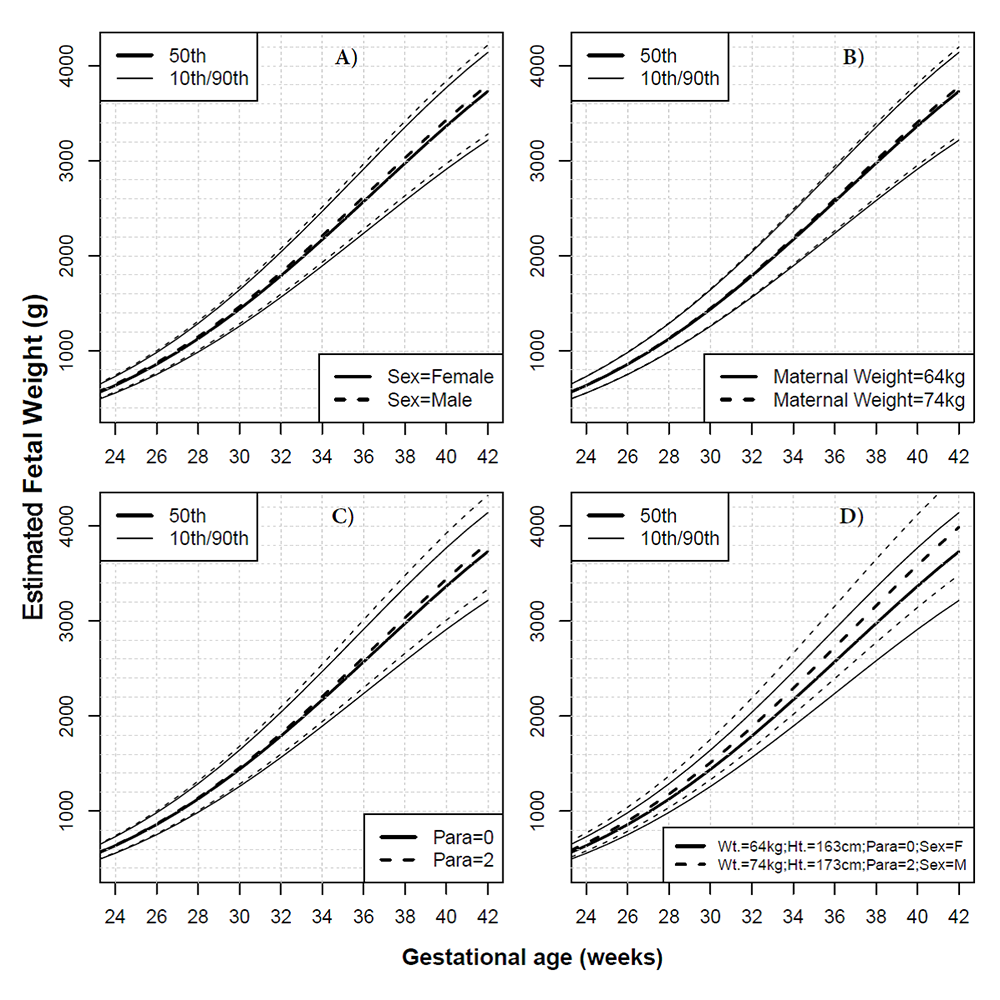
Figure 3. Effect of covariates on fetal growth in African American women
Unless otherwise stated in the legend of each panel, continuous lines represent the estimated fetal weight median and the 10th/90th centiles for a female (F) fetus born at term to a nulliparous African American mother, having a height of 163 cm, weighing 64 kg at first visit. Interrupted lines show how the chart would change for a male fetus (A), for 10 additional kg of maternal weight (B), for a mother in her third pregnancy (parity=2) (C), and for a combination of factors (10 additional kg in maternal weight [Wt.], 10 additional centimeters in height [Ht.], parity of 2, and a male [M] fetus) (D).
The screen-positive rate for SGA was 7.2% for the NICHD African American standard, 12.3% for the GROW standard, 13% for the WHO standard customized by fetal sex, and 14.4% for the PRB/NICHD customized standard. For all standards, the screen-positive rate for SGA was at least two-fold higher among fetuses delivered preterm than at term. The screen-positive rate for LGA was 8.7% for the GROW standard, 9.2% for the PRB/NICHD customized standard, 10.8% for the WHO standard customized by fetal sex, and 12.3% for the NICHD African American standard. Finally, the highest overall agreement among standards was between the GROW and PRB/NICHD customized standards.
We developed a novel customized PRB/NICHD fetal growth standard from fetal data in an African American population without assuming proportionality of the effects of covariates, and without assuming that these effects are equal on all centiles of weight; we also provide an easy-to-use centile calculator. The standard classified more fetuses as being at risk for SGA than do existing standards, especially among fetuses delivered preterm, but it classified about the same number of LGA. The comparison among the four growth standards also revealed that the most important factor determining agreement among standards is whether they account for the same factors known to affect fetal growth.
Fetal intelligent navigation echocardiography (FINE) detects 98% of congenital heart disease [Reference 5].
FINE is a novel sonographic method that we invented and that automatically generates and displays nine standard fetal echocardiography views in normal hearts by applying "intelligent navigation" technology to spatiotemporal image correlation (STIC) volume datasets of the fetal heart. We set out to determine the sensitivity and specificity of FINE in the prenatal detection of congenital heart disease (CHD). We conducted a case-control study of 50 fetuses with a broad spectrum of CHD (cases) (Figure 4) and 100 fetuses with normal hearts (controls) in the second and third trimesters. Using 4-dimensional ultrasound with STIC technology, we acquired volume data sets. After all identifying information was removed, the data sets were randomly distributed to a different investigator for analysis using the FINE method. The diagnostic performance of FINE for the prenatal detection of CHD was: sensitivity of 98% (49 of 50), specificity of 93% (93 of 100), positive likelihood ratio of 14, and negative likelihood ratio of 0.02. Among cases with confirmed CHD, the diagnosis with use of FINE completely matched the final diagnosis in 74% (37 of 50); minor discrepancies were seen in 12% (6 of 50), and major discrepancies were seen in 14% (7 of 50). This is the first time that the sensitivity and specificity of FINE in fetuses with normal hearts and CHD in the second and third trimesters have been reported. Because FINE identifies a broad spectrum of CHD with 98% sensitivity, the method could be used prenatally to screen for and diagnose CHD.
Click image to view.
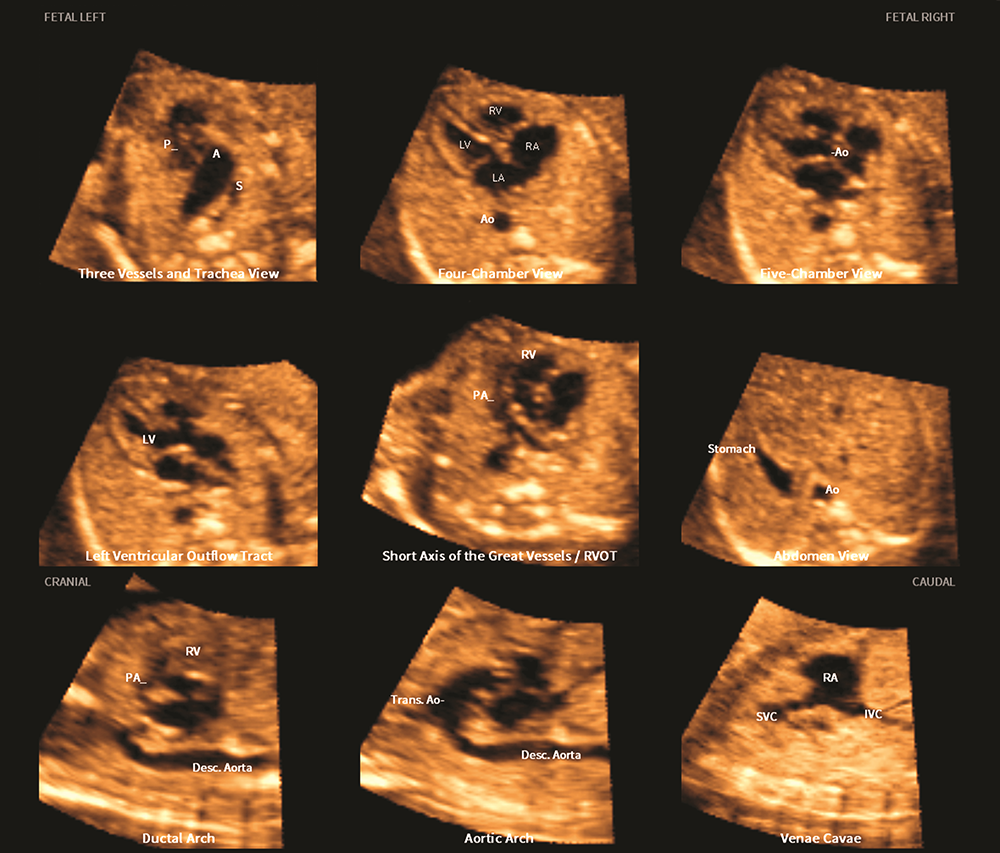
Figure 4. Application of the FINE method to a fetus with tetralogy of Fallot at 23 weeks of gestation
The six echocardiography views are abnormal and demonstrate the typical features of this cardiac defect.
Publications
- Romero R, Conde-Agudelo A, Da Fonseca E, O'Brien JM, Cetingoz E, Creasy GW, Hassan SS, Nicolaides KH. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol 2018;218(2):161-180.
- Conde-Agudelo A, Romero R, Da Fonseca E, O’Brien JM, Cetingoz E, Creasy GW, Hassan SS, Erez O, Pacora P, Nicolaides KH. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol 2018;219(1):10-25.
- Frascoli M, Coniglio L, Witt R, Jeanty C, Fleck-Derderian S, Myers DE, Lee TH, Keating S, Busch MP, Norris PJ, Tang Q, Cruz G, Barcellos LF, Gomez-Lopez N, Romero R, MacKenzie TC. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci Transl Med 2018;10(438):2263.
- Tarca AL, Romero R, Gudicha DW, Erez O, Hernandez-Andrade E, Yeo L, Bhatti G, Pacora P, Maymon E, Hassan SS. A new customized fetal growth standard for African American women: the PRB/NICHD Detroit study. Am J Obstet Gynecol 2018;218:S679-S691.
- Yeo L, Luewan S, Romero R. Fetal intelligent navigation echocardiography (FINE) detects 98% of congenital heart disease. J Ultrasound Med 2018;37(11):2577-2593.
Collaborators
- Tinnakorn Chaiworapongsa, MD, Wayne State University School of Medicine, Detroit, MI
- Agustin Conde-Agudelo, MD, Wayne State University School of Medicine, Detroit, MI
- Mark Haacke, PhD, Wayne State University School of Medicine, Detroit, MI
- Sonia Hassan, MD, Wayne State University School of Medicine, Detroit, MI
- Edgar Hernandez-Andrade, MD, Wayne State University School of Medicine, Detroit, MI
- Chong-Jai Kim, MD, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
- Leonid Margolis, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Adi L. Tarca, PhD, Wayne State University, Detroit Medical Center, Detroit, MI
- Moriah Thomason, PhD, Wayne State University School of Medicine, Detroit, MI
- Lami Yeo, MD, Wayne State University School of Medicine, Detroit, MI
- Bo Hyun Yoon, MD, PhD, Seoul National University, Seoul, Korea
Contact
For more information, email romeror@mail.nih.gov or visit irp.nih.gov/pi/roberto-romero.


