Translational Biophotonics in Developmental Disorders and Diseases

- Amir H. Gandjbakhche, PhD, Head, Section on Translational Biophotonics
- Fatima A. Chowdhry, MD, Research Fellow
- Afrouz Anderson, PhD, Postdoctoral Fellow
- Emma Condy, PhD, Postdoctoral Fellow
- Helga De Oliveira Ramirez, PhD, Postdoctoral Fellow
- Kosar Khaksari, PhD, Postdoctoral Fellow
- Siddharth Khare, PhD, Postdoctoral Fellow
- Hadis Dashtestani, MS, Intramural Research Training Award Student
- Neel Bhardwaj, BS, Postbaccalaureate Fellow
- Aisling Casey, BS, Postbaccalaureate Fellow
- Hye Soo Chun, BS, Postbaccalaureate Fellow
- Joy Cui, BS, Postbaccalaureate Fellow
- Douglas Harrison, BS, Postbaccalaureate Fellow
- Siamak Aram, PhD, Guest Researcher
- Yasaman Ardeshirpour, PhD, Guest Researcher
Brain imaging and spectroscopy of developmental disorders
Functional near-infrared spectroscopy (fNIRS) is a non-invasive and wearable imaging technique that assesses brain function and is suitable for studies of children and toddlers, especially those with neuro-developmental disorders. Such measurements are based on local changes in the cerebral hemodynamic response associated with brain activity. NIR light (700–900 nm) can penetrate deep enough through tissue to probe the cortical region. The NIR absorption spectrum of tissue is sensitive to changes in the concentration of major tissue chromophores, such as hemoglobin. Therefore, measurements of temporal variation in backscattered light can capture functionally evoked changes in the cortex to assess brain function. We are currently pursuing two general tracks of research involving fNIRS in the brain: the developmental trajectories of cognitive abilities and the evaluation of fNIRS using cognitive tasks that are used in fMRI.
In our previous studies, we used fNIRS to examine prefrontal cortical activation as it relates to developmental level in toddlers [Reference 1]. We have continued this work through a series of projects and collaborations. First, as part of a previous collaboration with Audrey Thurm, we designed a study in which 24-month-old toddlers listened to speech sounds or watched gesture production while we recorded fNIRS in the prefrontal cortex (PFC). Over the past year, we analyzed the data from this project, and manuscripts are currently under review or being published. As a continuation of this work, we began another project that will examine the developmental trajectory of the mirror neuron network (MNN) in infants. The MNN is associated with the development of sophisticated social behaviors that emerge in typical infants. By modeling MNN development, we hope to uncover a sensitive measure of deviations in social communication development before clinical behavioral deficits can be detected. MNN activation has been indicated through mu rhythm suppression using EEG. In our pilot study, we are recruiting healthy adults (n=40) to determine whether MNN activation can be elicited, using a motor observation and a simultaneous execution paradigm and EEG/fNIRS systems. We will then examine the synchronicity of these signals as they relate to social communication and cognitive functioning. Upon completion of the adult pilot, we intend to recruit typically developing infants (n=60) and infants at risk for developmental delays (n=60) from 9–12 months of age to collect fNIRS/EEG signals during the motor observation and the execution paradigm. At-risk infants will be brought in again at 24 months of age to evaluate any deviations in their social communicative development. We will examine their developmental status at 24 months in relation to their initial neural data to determine whether MNN activation can predict developmental outcomes. We aim to begin recruiting infants for this phase of the project in January 2019.
In a collaboration with Andrea Gropman, we are also examining brain function in children and adults with urea cycle disorders (UCD). UCDs are a set of rare genetic disorders caused by the loss of enzymatic activities (such as ornithine transcarbamylase deficiency [OTCD]) that convert ammonia to urea through the transfer of nitrogen. UCD often results in life-threatening hyperammonemia, resulting in a broad range of neurological impairments in working memory and executive function. Using functional magnetic resonance imaging (fMRI), Gropman’s studies found that patients with OTCD show impairments in frontal-lobe processing through their performance on a working memory task, compared with a control group. We are replicating this work using fNIRS. We recorded hemodynamic activity from the prefrontal cortex of 26 children and adults (control and UCD) while they performed N-back tasks (which test processing speed) and Stroop tasks (which evaluate executive functions). Data collection for this project is ongoing, with a goal of examining 40 subjects.
Click image to view.
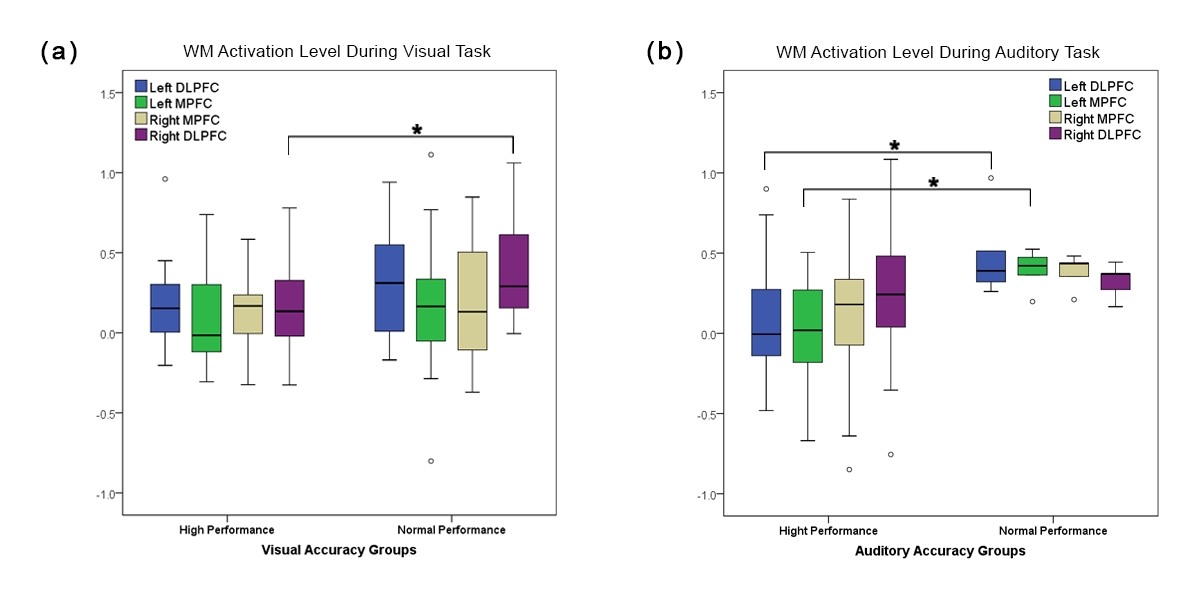
Figure 1. Differences in activation between high-performance (HP) and normal-performance (NP) groups
Both auditory and visual NP groups showed higher activation in the right dorsolateral PFC (DLPFC) during visual (a) and left DLPFC and medial PFC (MPFC) during auditory (b) tasks than did their HP cohorts.
We also used fNIRS to examine working memory in typically developing adults. Our results reveal that individual differences in learning style and performance can affect the lateralization of prefrontal cortex activation during the execution of a working memory task [Reference 2] (Figure 1). In another study, we implemented a moral judgment (MJ) task that is based on a series of questions in which subjects examine personal (emotionally salient) versus impersonal (less emotional and more logical) dilemmas. In two recently published studies [References 3 and 4], we investigated hemodynamic patterns for each dilemma and their correlation with psychopathic traits, as measured by the Psychopathic Personality Inventory-Revised Content Scale (PPI-R-CS). We analyzed fNIRS data using a non-linear classification method called a cubic support vector machine (SVM). We found that brain activity differs significantly during personal and impersonal dilemmas (mean accuracy of 85%). Consistent with fMRI studies, we found that left dorsolateral PFC is highly activated when subjects make non-utilitarian decisions (i.e., benefiting the majority group) for impersonal moral dilemmas. Our results using Canonical Correlation Analysis (CCA) showed a significant correlation between PFC activation and psychopathic traits, as measured by the PPI-R-CS. Specifically, cold-heartedness and carefree non-planfulness were highly correlated with PFC activation during personal moral dilemmas. Machiavellian egocentricity, rebellious nonconformity, cold-heartedness, and carefree non-planfulness are the core traits that exhibit similar dynamics with PFC activation during impersonal (more logical) moral dilemmas. Activation in ventromedial prefrontal cortex (vmPFC) and left lateral PFC were positively correlated with PPI-R-CS traits during personal dilemmas; on the other hand, the right vmPFC and right lateral PFC show positive correlation during impersonal dilemmas (Figure 2).
Click image to view.
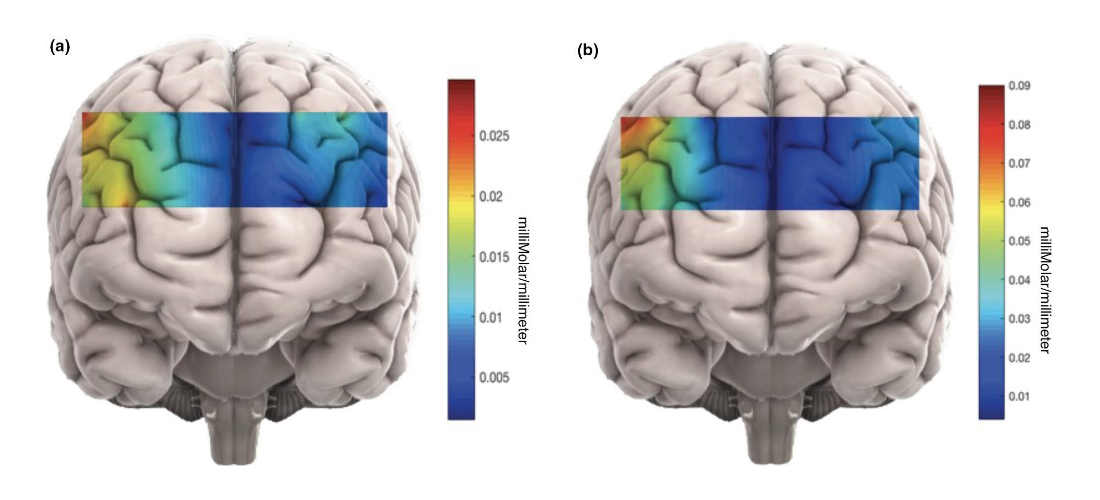
Figure 2. Changes in mean HbO during personal and impersonal MJ tasks.
Changes in mean HbO which have been approximately mapped on different brain regions during (a) personal and (b) impersonal moral judgment (MJ) tasks. The captured brain activity during impersonal scenarios was significantly higher than during personal dilemmas. The average hemodynamic change in the left DLPFC for impersonal dilemmas was especially large. HbO: oxyhemoglobin.
Additionally, we conducted a study to examine neural activation during a go/no-go behavior inhibition task that activates prefrontal cortical areas. The go/no-go task was administered to 44 typically developing adults while fNIRS and heart rate were recorded. We found that fNIRS detected differences between baseline and the go/no-go task and could be a suitable alternative to fMRI in the evaluation of behavior inhibition. We are further analyzing the data to determine whether fNIRS measurements are related to individuals’ level of task performance or to more general measures of day-to-day behavior inhibition abilities. Analysis is ongoing, with the goal of submitting a manuscript by the end of 2018.
Tissue characterization and function
We are investigating photonic techniques to elucidate biomarkers for the diagnosis of disease or the assessment of treatment outcome across a variety of conditions. Our first study is in the assessment of facial plethora in Cushing’s syndrome (CS), as it is one of the earliest described clinical features of the disease. In collaboration with Constantine Stratakis, we quantified changes of facial plethora in CS as an early assessment of cure. We performed non-invasive multi-spectral NIR imaging on the right cheek of patients before and after surgery. Patients were defined as cured by post-operative measurements of plasma cortisol less than 3 (mcg/dl) and/or adrenocortical insufficiency, for which they received replacement therapy. Results indicate that a reduction in facial plethora after surgery, as evidenced by decrease in blood volume fraction, is correlated with the cure of CS. The first set of results were published in 2015 [Afshari A et al., J Clin Endocrinol Metab 2015;100:3928]. We also showed that water content fraction could be used as a new biomarker of early cure in patients with CS. We are pursuing the technique to assess Kaposi Sarcoma (KS) in three ongoing NCI clinical trials. Given the capability of our technology, the goal is to further evaluate diffuse multispectral imaging in a relatively large sample as a potential supplement to existing response assessment in KS. Previously, we showed that successful treatment is indicated by a reduction in the blood volume in lesions, making this method a quantitative marker of tumor response to therapy. As a next step in our 'bench-to-bedside' goal, we developed a new hand-held multispectral camera to be used as a point-of-care system. The device uses a high-resolution complementary metal-oxide semiconductor (CMOS) camera with on-chip filters. High-resolution images are acquired simultaneously at eight different near-infrared wavelengths (700–980 nm). We also developed a user-friendly graphical interface for data processing.
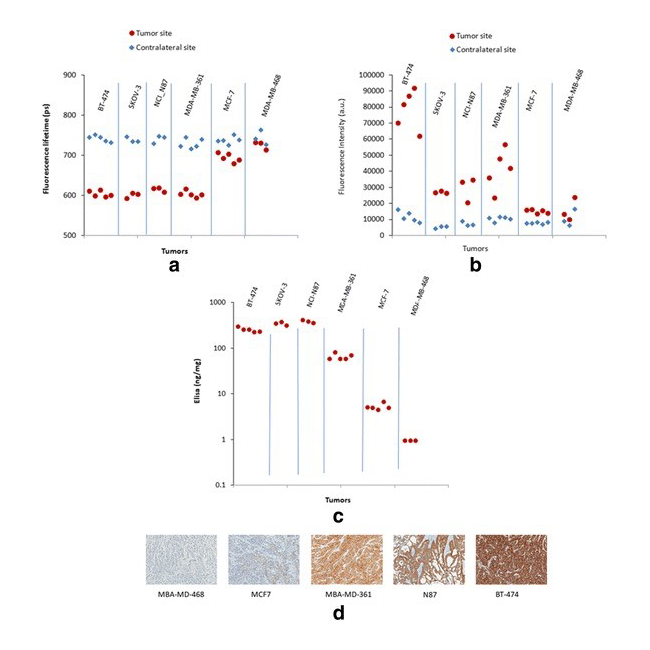
We are also investigating the assessment of tumor development in patients with the goal of facilitating treatment strategies and identifying targets for early intervention. In our recently published study [Reference 5], we designed time-resolved fluorescence lifetime imaging to distinguish bound human epidermal growth factor 2 (HER2)–specific affibody probes to HER2 receptors in live animals. Our results show that changes in fluorescence lifetime of the bound contrast agent can be used to rapidly assess the high- to mid-level expression of HER2–expressing tumors in vivo (Figure 3). This patient-friendly approach allows the use of only one measurement for diagnosis and determining the efficacy of treatment intervention.
Click image to view.
Figure 3. Fluorescence lifetime of imaging agent correlates with HER2 expression in cancer.
Results for different parameters for six tumor cell lines expressing various levels of HER2. Comparison between the tumor and the contralateral site. Fluorescence lifetime (a) and fluorescence intensity (b) measured in vivo at the tumor and contralateral sites, 1 h after injection of HER2-specific Affibody probe. ELISA results in (c) and (d).
As a continuation of our work in preterm pregnancy complications, in collaboration with Jessica Ramella-Roman, we used the Preterm Imaging system based on colposcopy to characterize uterine cervix structure in a longitudinal study of low-risk and high-risk (i.e., prior preterm birth [PTB] or a sonographic short cervix) patients [Reference 6]. Polarization imaging is an effective tool to measure optical anisotropy in birefringent materials, such as the cervix's extracellular matrix, and to predict cervical ripening. For this reason, it has potential to predict pre-term birth. Through our collaboration with Roberto Romero’s Branch and Ramella-Roman, we will test the system in a control population and those with PTB prevalence.
Placenta oxygenation from basics to point of care
Monitoring placenta oxygenation is critical to ensure a healthy pregnancy outcome. Abnormalities in placental oxygenation have been associated with preeclampsia, intrauterine growth restriction, fetal hypoxia, and cerebral palsy. Therefore, it is crucial to have a quantitative understanding of placental oxygenation. In designing a placental oximeter, the following criteria should be prioritized: it must be light-weight, relatively small, and battery operated and have wireless capability. Most importantly the device must be relatively inexpensive, so it can be used in low-resource settings, where the most high-risk cases are to be expected. fNIRS is a convenient technique for dynamic in vivo monitoring of anterior placental oxygenation. In parallel to the in vivo studies, we investigate the placenta at the cellular level to study the effect of oxygen level on cell metabolism. We intend to first find the baseline placental oxygenation for normal pregnancies to and standardize the oxygenation data across pregnancies, and second to study placental cell metabolism in vitro at physiologically relevant variations of oxygen level, using Dynamic Full Field Optical Coherence Tomography (DFFOCT).
For the in vivo study, we created a fast, non-invasive, and wearable device to allow continuous measurement of the oxygenation of the anterior placenta in a subject-friendly environment. The light-weight compact system can be positioned at various abdominal locations for localized measurement of oxygenation. The NIRS device uses light in near infrared region (750 and 850 nm) and consists of several source-detector pairs to simultaneously probe maternal and placental tissue. We investigated the efficiency of the device in separating oxygenation of the maternal and placental tissue while accounting for variations in melanin concentration and fat content. We developed a multi-layer model based on Monte Carlo simulation that includes the optical properties of skin, fat, uterus, and placental tissue. We further measured the optical properties of the placenta using dual wavelength LED sources with a photodiode array unit, which we built in-house, to calculate the attenuation coefficient (as a function of the scattering and absorption coefficients) based on the diffuse reflection curve from placental tissues. Using the above methods, we developed a system that includes parameters such as skin color and fat thickness in the calculation of oxygenation index.
In collaboration with the Sonia Hassan, Shad Deering, and Ramon Diaz-Arrastia, we are testing our device in pilot studies. In our first pilot study, we are measuring the oxygenation of the placenta during the last trimester in normal pregnancies to establish the baseline placental oxygenation. Meanwhile, we are continuing to refine our data analysis software by incorporating anatomical data from subjects. By taking advantage of electronic miniaturization of spectroscopy, along with Artificial Intelligence for the classification of data between typical and atypical pregnancies, we expect to provide earlier detection of pregnancy complications, which can improve maternal and fetal health in the future.
For the in vitro study, we are piloting an experiment to confirm DFFOCT’s ability to capture metabolically coupled movements in in vitro samples. We are comparing the images of cells cultured at physiological hypoxia and hyperoxia with DFFOCT to detect the difference in the dynamics and using protein expression to verify altered cellular metabolism. To accurately maintain the oxygen level, we pass pre-equilibrated media over the sample while imaging. We will statistically analyze the recorded data to validate the findings. Through this study, we expect to establish an understanding of placental metabolism under varying oxygenation states.
Additional Funding
- Bench to Bedside Award 345 (2016): "Mirror neuron network dysfunction as an early biomarker of neurodevelopment" (Ongoing)
- Human Placenta Project-NICHD (2016) (Ongoing)
Publications
- Anderson AA, Smith E, Chowdhry FA, Thurm A, Condy E, Swineford L, Manwaring SS, Amyot F, Matthews D, Gandjbakhche AH. Prefrontal hemodynamics in toddlers at rest: a pilot study of developmental variability. Front Neurosci 2017;11:300-310.
- Anderson AA, Parsa K, Geiger S, Zaragoza R, Kermanian R, Miguel H, Dashtestani H, Chowdhry FA, Smith E, Aram S, Gandjbakhche AH. Exploring the role of task performance and learning style on prefrontal hemodynamics during a working memory task. PloS One 2018;13(6):e0198257.
- Dashtestani H, Zaragoza R, Kermanian R, Knutson KM, Halem M, Casey A, Shahni Karamzadeh N, Anderson AA, Boccara AC, Gandjbakhche A. The role of prefrontal cortex in a moral judgment task using functional near infrared spectroscopy. Brain Behav 2018;8(11):e01116.
- Dashtestani H, Zaragoza R, Pirsiavash H, Knutson KM, Kermanian R, Cui J, Harrison JD Jr, Halem M, Gandjbakhche A. Canonical correlation analysis of brain prefrontal activity measured by functional near infra-red spectroscopy (fNIRS) during a moral judgment task. Behav Brain Res 2018;359:73-80.
- Ardeshirpour Y, Sackett DL, Knutson JR, Gandjbakhche AH. Using in vivo fluorescence lifetime imaging to detect HER2-positive tumors. EJNMMI Res 2018;8(1):26.
- Chue-Sang J, Holness N, Gonzalez M, Greaves J, Saytashev I, Stoff S, Gomes J, Jung R, Gandjbakhche A, Chernomordik VV, Burkett G, Ramella-Roman JC. Use of Mueller matrix colposcopy in the characterization of cervical collagen anisotropy. J Biomed Opt 2018;23:1-9.
Collaborators
- Franck Amyot, PhD, Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD
- Claude Boccara, PhD, École Supérieure de Physique et de Chimie Industrielles, Paris, France
- Shad Deering, MD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Ramon Diaz-Arrastia, MD, PhD, Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD
- Andrea Gropman, MD, Children's National Health System, Washington, DC
- Sonia S. Hassan, MD, Wayne State University School of Medicine, Detroit, MI
- Jay Knutson, PhD, Laboratory of Molecular Biophysics, NHLBI, Bethesda, MD
- Maya Lodish, MD, Pediatric Endocrinology Inter-Institute Training Program, NICHD, Bethesda, MD
- Tom Pohida, MS, Division of Computational Bioscience, Center for Information Technology, NIH, Bethesda, MD
- Randall Pursley, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Jessica C. Ramella-Roman, PhD, Florida International University, Miami, FL
- Roberto Romero-Galue, MD, Perinatology Research Branch, NICHD, Detroit, MI
- Dan Sackett, PhD, Division of Basic and Translational Biophysics, NICHD, Bethesda, MD
- Babak Shadgan, MD, MSc, PhD, University of British Columbia, Vancouver, Canada
- Constantine Stratakis, MD, D(med)Sci, Section on Endocrinology and Genetics, NICHD, Bethesda, MD
- Audrey Thurm, PhD, Pediatrics & Developmental Neuropsychiatry Branch, NIMH, Bethesda, MD
- Eric Wassermann, MD, Cognitive Neuroscience Section, NINDS, Bethesda, MD
- Robert Yarchoan, MD, HIV and AIDS Malignancy Branch, NCI, Bethesda, MD
Contact
For more information, email amir@helix.nih.gov or visit http://safb.nichd.nih.gov.


