The Biophysics of Protein-Lipid Interactions in Influenza, Malaria, and Muscular Dystrophy

- Joshua Zimmerberg, MD, PhD, Head, Section on Integrative Biophysics
- Paul S. Blank, PhD, Staff Scientist
- Svetlana Glushakova, MD, PhD, Staff Scientist
- Matthias Garten, PhD, Visiting Fellow
- Sourav Haldar, PhD, Visiting Fellow
- Brad Busse, PhD, Postdoctoral Intramural Research Training Award Fellow
- Chad McCormick, PhD, Postdoctoral Intramural Research Training Award Fellow
- Ludmila Bezrukov, MS, Chemist
- Hang Waters, MS, Biologist
- Elena Mekhedov, MA, Contractor
- Tatyana I. Tenkova-Heuser, PhD, Contractor
- Glen Humphrey, PhD, Guest Researcher
- John E. Heuser, MD, Senior Biophysicist
- Jennifer Petersen, PhD, Electron Microscopist
- Katherine Chang, BA, Postbaccalaureate Fellow
- Alexandra Sjaarda, BA, Postbaccalaureate Fellow
Fusion and fission, the instances when organelles gain or lose their identities, are the essence of complex membrane dynamics in living cells and are key elements of synapses and other dynamic cellular trafficking networks. Without fusion and fission, enveloped viruses and parasites could not enter cells, replicate, or exit cells, nor would inflammatory cells respond and kill such invaders or deal with sick cells. Our earliest work concentrated on model membrane systems, the physical properties and theoretical pathways required for membrane fusion to occur, and the discovery that tension spreads headgroups for hemifusion, then pulls open fusion pores to allow coalescence of adherent bilayers. However, while able to focus on basic membrane biophysical properties and help develop a theoretical framework for understanding membrane interactions, model systems were a simplification that ignored the important roles of proteins. Including the role of proteins in these fundamental biophysical processes was both fruitful and informative, culminating in what we believed to be a canonical framework for understanding both fusion and fission. We introduced a simple paradigm: proteins act as catalysts (bilayer topoisomerases) for lowering the huge energy barriers to membrane remodeling steps. A few amino acids of a specialized protein domain can reversibly enter the hydrophobic membrane matrix or cover the headgroups as inclusions or scaffolds, respectively, and thus transiently alter the thermodynamics of the system by specific protein-lipid interactions. By combining quantitative light microscopy with electrophysiology, and reconstitution of fusion and fission in lipid bilayer membranes, we constructed hypotheses with predicted fusion intermediates whose dimensions were deduced by continuum theory and fits to experiments. The predicted sizes were detectable by cryo-electron microscopy, so we labored to achieve the highest-resolution electron microscopy of hydrated membrane fusion events in order to understand how proteins catalyze the new configurations of lipids that ultimately mediate these processes. By successfully installing a new technology at NIH, the Volta Phase Plate, we were able to visualize the predicted hemifusion diaphragm mediated by the hemagglutinin (HA) of influenza virus (IFV), and the measurements of its dimensions fit the predictions of continuum theory. However, another result was unexpected: HA catalyzed the breakage of membranes, leading to free membrane edges—often in great profusion.
To understand why this was unexpected, one must consider the physical forces that act on lipids in solution. Membranes avoid edges. The lipid bilayer is self-assembling because its free energy of cohesion (which derives in part from enthalpic attractive forces between hydrocarbon chains and in part from the entropic hydrophobic effect that minimizes interfacial area) automatically ensures stability of the lipid bilayer. Formally, the edge of an otherwise lamellar membrane has a large linear tension, i.e., should be a high-energy region that the membrane seeks to minimize. Nevertheless, we observe that 'free edges' do indeed outnumber hemifusion diaphragms for certain lipid compositions of target membranes. Such edges only occur in close vicinity to activated HA molecules, indicating that edges are triggered to form by the same event that triggers full fusion: namely, the amphipathic helix of HA being ejected from HA and binding to the target bilayer. We can only presume for the moment that the HA fusion peptide somehow stabilizes the observed membrane edges, i.e., drastically lowers bilayer line-tension. This observation and resultant hypothesis forms the basis of our future work on the influenza virus. At the same time, we are (1) actively investigating the interaction of proteins and lipids in regulating the membrane-remodeling stages of the malaria parasite, to better understand how to produce improved anti-malarial compounds, and (2) trying to determine the relationship between human subject activity and the leakage of cytosolic components of skeletal muscle into the blood stream for individuals with fragile-membrane muscular dystrophies.
Membrane fusion, fission, and enzyme phosphorylation in the pathophysiology of influenza and insulin resistance
There are two threads that run through all of the Section’s work: the use of quantitative measurements of dynamic systems to test hypotheses deduced from biophysical reasoning. But our impact goes beyond our single experiment when we develop new technology that paves the road to future work. In the past, capacitance measurements opened up the field of the fusion pore to experimentation, as did simultaneous imaging with electro-physiology, and laser excitation of photo-activatable fluorophores opened up single-molecule imaging to living cells. More recently, the implementation of electrical and fluorescent measurements of cylindrical lipid nanotubes allowed us to test hypotheses regarding the interaction of dynamin and membrane curvature–scaffolding proteins with membranes. We found that the GTPase dynamin acts as a scaffold with many small amino acid insertions into the outer monolayer of a lipidic structure called the fission pore, to form a hemi-fission intermediate. The hemi-fusion intermediate was induced by influenza virus hemagglutinin (HA) incorporating in virosomes and interacting with targeted lipid vesicles at low pH. Ultra-thin films were plunge-frozen and visualized by Volta phase plate cryo-electron tomography (VPP-cET). We identified two distinctly different hemifusion structures: a hemifusion diaphragm and a highly unexpected novel structure termed a 'lipidic junction.' The edges of liposomes' lipidic junctions were ruptured and stabilized by HA. The high frequency of lipidic junctions exclude their artefactual origin. Both rupture frequency and hemifusion diaphragm diameter declined when the liposome cholesterol level matched physiological concentrations. In a separate study, we showed that the acylation of the cytosolic tail of HA changes membrane curvature [Reference 1].
Lipid-dependence of target membrane stability during influenza viral fusion
Although influenza kills about a half million people every year, even after excluding pandemics, there is only one set of antiviral drugs: the neuraminidase inhibitors. By using a new approach utilizing giant unilamellar vesicles and infectious X-31 influenza virus, and testing for the newly identified pore intermediate of membrane fusion, we observed about 30–87% poration, depending upon lipid composition. Testing the hypothesis that spontaneous curvature (SC) of the lipid monolayer controls membrane poration, our Poisson model and Boltzmann energetic considerations suggest a transition from a leaky to a non-leaky fusion pathway depending on the SC of the target membrane [Reference 4]. When the target membrane SC is below approximately −0.20 nm−1, fusion between influenza virus and target membrane is predominantly non-leaky, whereas above that, fusion is predominantly leaky, suggesting that influenza HA–catalyzed topological conversion of target membranes during fusion is associated with a loss of membrane integrity.
Subcutaneous adipose tissue imaging of human obesity reveals two types of adipocyte membranes: insulin-responsive and non-responsive.
In adipose tissue, resistance to insulin’s ability to increase glucose uptake can be induced by many factors, including obesity. Impairment of insulin action may occur at various spatial loci at the cellular or subcellular level. To begin to understand the spatial response to insulin in human subcutaneous adipose tissue (hSAT), we continued to develop our quantitative imaging method for activation of a major signaling node in the gluco-regulatory insulin signaling pathway. After treatment with insulin or control media, we immuno-stained biopsied tissues for Akt phosphorylation at Thr308/9 (pAkt) and then imaged by confocal fluorescence microscopy automated to collect a large grid of high-resolution fields. In hSAT from 40 obese men and women, substantial heterogeneity of pAkt densities in adipocyte membranes were quantified in each image mosaic, using a spatial unit of at least twice the size of the point-spread function. Statistical analysis of the distribution of pAkt spatial units was best fit as the weighted sum of two separate distributions, corresponding to either a low or high pAkt density. A "high–pAkt fraction" metric was calculated from the fraction of high–pAkt distributed units over the total units. Importantly, upon insulin stimulation, tissues from the same biopsy showed either a minimal or a substantial change in the high–pAkt fraction. Further supporting a two-state response to insulin stimulation, subjects with similar insulin sensitivity indices are also segregated into either of two clusters identified by the amount of membrane-localized pAkt.
Membranes during egress of Plasmodium falciparum, the causative agent of malaria
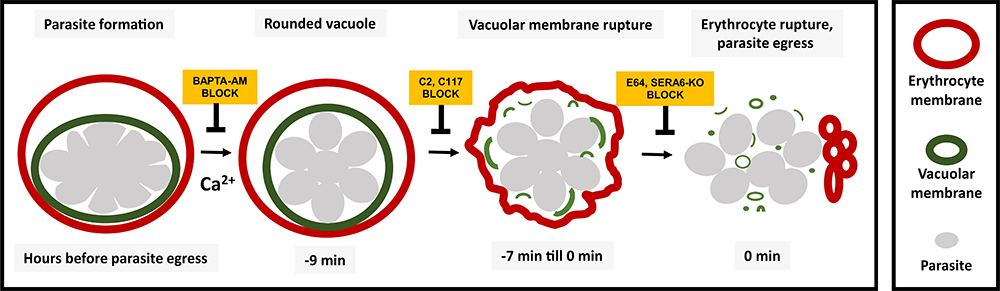
Click image to view.
Figure 1. Sequence of events leading to release of malaria parasite from red blood cells
Diagram showing the sequence of events involved in rupture of the vacuole and cell membrane. Using chemical inhibitors, we showed that it is possible to block each event in the sequence.
BAPTA-AM: 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid acetoxymethyl ester, a cell-permeable Ca2+ chelator; C2: compound 2, or 4-[7-[(dimethylamino)methyl]-2-(4-fluorphenyl)imidazo[1,2-α]pyridine-3-yl]pyrimidin-2-amine); CWHM-117: compound 8p; E-64: thiol protease inhibitor; SERA-6 KO: knock out of the SERA 6 gene.
We focus on our continued research on the physiology of the deadly malaria parasite Plasmodium falciparum. Despite some progress in the combating malaria, Plasmodium spp. cause over 200 million annually reported malaria cases with more than 400,000 deaths, mostly in children under the age of 5. With no vaccine, and drug resistance rising, we are focusing on the unique membrane biology of the parasites to find new targets for therapy. By developing, publishing, and promulgating new methods to study the biology of the malaria parasite, our work has impacted the field by transforming qualitative imaging to quantitative measures, by providing, e.g., the first recordings of P. falciparum egress and invasion of erythrocytes, and by describing new phenomena such as shape transformation of infected cells, which signals the egress initiation and membrane transformation upon egress. We developed several non-interventional methods that permit fine-staging of cell phenotype and quantification of the parasite replication cycle, as it naturally progresses from parasite invasion of erythrocytes to parasite egress from the host cells.
Last year, we reported a pair of 'druggable' mediators of parasite egress and invasion, namely, aspartic proteases Plasmepsins IX and X. We determined that the aspartic protease PMIX (acting from within the 'rhoptry,' apical secretory organelles of Plasmodia) is essential for erythrocyte invasion [Reference 2]. In contrast, by controlling maturation of the subtilisin-like serine protease SUB1 in exoneme secretory vesicles, PMX is essential for both egress and invasion. A lead compound, C-117, is currently under intense evaluation by pharmaceutical companies, because it works in the high nanomolar range and is well tolerated orally by mice. In another study, we showed a new potential route for drug delivery. Despite its membrane impermeability, heparin, a natural glycosaminoglycan, inhibited malaria parasite egress, trapping merozoites within infected erythrocytes. Heparin does not bind to the erythrocyte surface, but rather enters the infected red blood cell (iRBC) at the last minute of the parasite cycle through parasite-induced pores that we discovered in iRBC. This short encounter is sufficient to significantly inhibit parasite egress and dispersion. Heparin blocks egress by interacting with both the surface of merozoites and the inner aspect of erythrocyte membranes, preventing the rupture of infected erythrocytes but not of parasitophorous vacuoles, and independently interfering with merozoite disaggregation. Given that this action of heparin offers a plausible explanation for how neutralizing antibodies can block egress, we intend to exploit membrane perforation as a new physiological strategy to target therapeutics intracellularly.
EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX.
The blood stage of the parasite is responsible for the symptoms of malaria. Understanding how the parasite establishes a red blood cell infection and acquires nutrients is critical to devise new ways to combat a parasite that repeatedly developed resistance to frontline treatments. Blood-stage malaria parasites reside within a parasitophorous vacuolar membrane (PVM); PVMs form when parasites invade their host cell. Establishment of infection requires the parasite to export effector proteins into the red blood cell cytosol, as well as to import nutrients past the PVM. Protein export is achieved by a protein complex, the Plasmodium translocon of exported proteins (PTEX). Its putative membrane-spanning pore complex consists of the protein EXP2, which shares sequence homology with nutrient-permeable pores of other apicomplexans, suggesting a potential dual role of the protein in nutrient uptake and protein export. Using regulated gene expression, we showed that EXP2 is essential for protein export [Reference 3]. Further, EXP2 expression correlates with the occurrence of a previously characterized nutrient-permeable PVM channel of unknown molecular identity in cell-attached patch-clamp experiments. To show that EXP2 indeed constitutes the nutrient-permeable PVM channel, charged amino acid residues of EXP2 were truncated, which diminished the response of the nutrient-permeable channel to applied voltages, thus identifying EXP2 as the channel-forming protein. The results put EXP2 in the center of focus for understanding nutrient import and protein export past the PVM in blood-stage malaria, and therefore how to disrupt it. The realization represents an important step in understanding the interaction of the malaria parasite with its host cell.
Rounding precedes rupture and breakdown of vacuolar membranes minutes before malaria parasite egress from erythrocytes.
Because Plasmodium falciparum replicates inside of a parasitophorous vacuole (PV) within a human erythrocyte, parasite egress requires the rupture of two limiting membranes. Parasite Ca2+, kinases, and proteases contribute to efficient egress; however, their coordination in space and time is not known. We linked the kinetics of parasite egress to specific steps with specific compartment markers, using live-cell microscopy of parasites expressing PV–targeted fluorescent proteins, and specific egress inhibitors [Reference 5]. Several minutes before egress, under control of parasite [Ca2+]I, the parasitophorous vacuole began rounding. Then after about 1.5 minutes, under control of PfPKG (Plasmodium falciparum cGMP–dependent protein kinase) and the transcriptional regulator SUB1, there was abrupt rupture of the PV membrane and release of vacuolar contents. Over the next approximately six minutes, simultaneously with erythrocyte membrane distortion, the vacuolar membrane progressively deteriorated, which lasted until the final minute of the egress program, when newly-formed parasites mobilized and erythrocyte membranes permeabilized and then ruptured—a dramatic finale to the parasite cycle of replication. The new stage discovered in this project has features that suggest the possibility of a new target for antimalarial drug development.
Developing methods to test activity and dietary regimen modification to treat fragile membrane muscular dystrophy
There are currently no routinely available, easily accessible, objective outcome measurements for either disease progression or treatment efficacy in the muscular dystrophies. A major theme under development for the past few years is that membrane transformations and dynamics, including the newly identified membrane "edge," are strongly influenced by lipid composition. To extend our research to physiology and pathophysiology, we explored the possibility that lipid composition can be altered with diet. We designed a dietary intervention trial in the dysferlin-deficient A/J mouse (a model of muscular dystrophy), which develops a mild myopathy after 6 months of age, to test whether a diet rich in alpha-linoleic acid alters lipid content by an iso-caloric substitution of flaxseed oil for soybean oil in a standard defined diet. Last year, we introduced proof-of-principle studies in dietary changes and mass spectrometry measurements of membrane lipids to advance our ability to study the effects of specific lipids on membrane stability and to test lipids as therapies in models of muscular dystrophy. A new assay for phytanic acid lipids was developed. With feeding, we succeeded in increasing the muscle content of this specific lipid in mice.
Towards a surrogate biomarker for efficacy in muscular dystrophies.
A major barrier to clinical trials for muscular dystrophy is the lack of a good measure of therapeutic efficacy. We are developing a time-series analysis procedure that uses event-driven, kinetic properties of high-frequency blood samples to reveal significant changes associated with minimal subject activity. Our approach showed that creatine kinase (CK), previously considered minimally informative because of large population variability, can be used as a biomarker when each subject is their own control. We further demonstrated that the amino transferases ALT and AST follow kinetics similar to that of CK and are significantly correlated. We predict that additional biomarkers exist and that their identification will facilitate the development of discriminants useful in the assessment of disease progression and/or treatment efficacy. We used the SOMAscan assay, a featured platform in the Center for Human Immunology (CHI), NIH, for the purpose of conducting novel proteomic biomarker discovery. For proof-of-principle and validation of the SOMAscan assay, we examined a subset of available samples representing blood collected before and after physical activity from the first visits of 10 subjects. Using an aptamer-based platform, we identified analytes in blood that significantly increased after the subjects arose from night-time sleep. As hypothesized, muscle-specific proteins (myoglobin and CK) were identified, confirming our earlier analyses based on clinical chemistry evaluations. In addition, we identified significant increases in signaling and chemokine proteins after arising. The results support our hypothesis that activity-correlated analyses is a viable procedure for identifying candidate biomarkers related to both muscle-specific membrane-damaging events and systemic physiological changes. We are in the process of extending our analyses; we believe that this newly developed procedure can aid in identifying disease progression and treatment efficacy.
Additional Funding
- 2018 Deputy Director for Intramural Research (DDIR) Innovator’s Award
- NIH Intramural-to-Russia (I-to-R) Program Award
- Office of AIDS Research (OAR) Award
Publications
- Chlanda P, Mekhedov E, Waters H, Sodt A, Schwartz C, Nair V, Blank PS, Zimmerberg J. Palmitoylation contributes to membrane curvature in Influenza A virus assembly and hemagglutinin-mediated membrane fusion. J Virol 2017;91:e00947.
- Nasamu AS, Glushakova S, Russo I, Vaupel B, Oksman A, Kim AS, Tolia N, Beck JR, Meyers MJ, Niles JC, Zimmerberg J, Goldberg DE. Plasmepsins IX and X are essential and druggable mediators of malaria parasite egress and invasion. Science 2017;358:518–522.
- Garten M, Nasamu AS, Niles JC, Zimmerberg J, Goldberg DE, Beck JR. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat Microbiol 2018;3:1090-1098.
- Haldar S, Mekhedov E, McCormick CD, Blank PS, Zimmerberg J. Lipid-dependence of target membrane stability during influenza viral fusion. J Cell Sci 2018;132:218321.
- Glushakova S, Beck JR, Garten M, Busse BL, Nasamu AS, Tenkova-Heuser T, Heuser J, Goldberg DE, Zimmerberg J. Rounding precedes rupture and breakdown of vacuolar membranes minutes before malaria parasite egress from erythrocytes. Cell Microbiol 2018;20:e12868.
Collaborators
- Oleg Batishchev, PhD, A.N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russia
- Josh Beck, PhD, Iowa State University, Ames, IA
- Nikki Curthoys, PhD, University of Maine, Orono, ME
- Andrew Demidowich, MD, PhD, Section on Growth and Obesity, NICHD, NIH, Bethesda, MD
- Rick M. Fairhurst, MD, PhD, Laboratory of Malaria and Vector Research, NIAID, Bethesda, MD
- Vadim Frolov, PhD, Universidad del País Vasco, Bilbao, Spain
- Daniel Goldberg, MD, PhD, Washington University, St. Louis, MO
- Samuel T. Hess, PhD, University of Maine, Orono, ME
- Mary Kraft, PhD, University of Illinois at Urbana-Champaign, Urbana, IL
- Richard Pastor, PhD, Membrane Biophysics, NHLBI, NIH, Bethesda, MD
- Thomas S. Reese, MD, Laboratory of Neurobiology, NINDS, Bethesda, MD
- Anna Shnyrova, PhD, Universidad del País Vasco, Bilbao, Spain
- Peter K. Weber, PhD, Lawrence Livermore National Laboratory, Livermore, CA
- Jack Yanovski, MD, PhD, Section on Growth and Obesity, NICHD, Bethesda, MD
Contact
For more information, email zimmerbj@mail.nih.gov or visit irp.nih.gov/pi/joshua-zimmerberg.


