Pathophysiology, Genetics, and Treatment of Congenital Adrenal Hyperplasia

- Deborah P. Merke, MD, MS, Adjunct Investigator, NICHD & Chief, Section of Congenital Disorders, CC
- Smita Jha, MD, Staff Clinician
- Ashwini Mallappa, MD, Associate Research Physician
- Qizong Lao, PhD, Staff Scientist
- Diala El-Maouche, MD, MS, Special Volunteer
- Vipula Kolli, PhD, Research Fellow
- Elizabeth Joyal, MSN, Nurse Practitioner
- Padmasree Veeraraghavan, RN, BSN, Clinical Nurse Specialist
- Ahmed Torky, MD, Pediatric Endocrine Training Program Fellow
- Brookner Brittany, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Alison R. Gaynor, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Kira Lokhmatova, BA, Postbaccalaureate Intramural Research Training Award Fellow
In its most severe classic form, congenital adrenal hyperplasia (CAH) is a life-threatening, rare orphan disease that is part of the neonatal screen performed in all 50 U.S. states. In its mildest non-classic form, CAH is considered one of the most common autosomal recessive diseases and may be a common cause of female infertility. Our intramural NIH research program strives to elucidate the pathophysiology and genetics of CAH, thus facilitating the development of new approaches to the diagnosis, evaluation, and treatment of the disease. We are conducting the largest ever Natural History Study of CAH, with over 450 patients enrolled. We were the first to identify adrenaline deficiency as a new hormonal imbalance in CAH and the first to report in CAH smaller-than-normal amygdala, the emotion regulator of the brain, providing insight into hormonal effects on the brain. We found that approximately 10 percent of patients with CAH due to 21-hydroxylase deficiency have a contiguous gene deletion syndrome resulting in CAH with a connective tissue dysplasia, Ehlers-Danlos syndrome, which represents a novel phenotype named CAH-X. Central to our work is the study of new treatments, including a long-term trial testing sex hormone blockade in children, and novel ways of replacing cortisol, aimed at mimicking the normal circadian rhythm of cortisol secretion. The NIH Clinical Center is the ideal venue in which to carry out these studies and is one of the few places in the world that facilitates the conduct of long-term studies of rare diseases.
Epidemiology of nonclassic congenital adrenal hyperplasia
The epidemiology of the classic or severe life-threatening form of CAH due to 21-hydroxylase deficiency is well established (1:10,000 to 1:20,000 live births) owing to the neonatal screening of millions of newborns in over 40 countries worldwide. Neonatal screening does not, however, accurately detect nonclassic CAH; data on the prevalence of the milder form of the disorder are therefore lacking. In a 1985 HLA-B linkage study of 210 families (43 families with nonclassic CAH), nonclassic CAH was estimated to be the most common autosomal recessive condition, with carrier rates especially high in Ashkenazi Jews. Surprisingly, similar studies had not been repeated. This past year, using state-of-the-art genetic analysis, we found that nonclassic CAH is less common in US Ashkenazi Jews than previously suggested and that nonclassic CAH is commonly found in the general US Caucasian population. In collaboration with the Johns Hopkins University School of Medicine's Epidemiology-Genetics Program, we genotyped 200 unrelated healthy subjects of Ashkenazi Jewish descent and 200 healthy Caucasians who did not self-identify as a specific ethnicity. Nonclassic CAH carriership was found in 15% of Ashkenazi Jews and 9.5% of Caucasians, and one subject in each cohort had a genotype consistent with being affected with nonclassic CAH [Reference 1].
As nonclassic CAH may result in infertility that is easily treated with glucocorticoid therapy and nonclassic CAH women not receiving glucocorticoid therapy might have higher miscarriage rates than those receiving treatment, our data have important implications for pre-conception and infertility counseling. Screening for this common and mostly undiagnosed condition in the setting of female infertility, regardless of ethnicity, would offer the opportunity for treatment.
Genotype-phenotype studies of CAH-X
CAH is most commonly caused by 21-hydroxylase deficiency. The gene encoding 21-hydroxylase, CYP21A2, and a highly homologous pseudogene, CYP21A1P, map to the short arm of chromosome 6 within the human leukocyte antigen histocompatibility complex. Deleterious sequence in the CYP21A1P pseudogene can be transferred to the CYP21A2 functional gene by homologous recombination, and such events produce common mutations that account for approximately 95% of all CYP21A2 disease–causing mutations. Of these common mutations, approximately 30% are large deletions. The TNXB gene encoding tenascin-X, an extracellular matrix protein that is highly expressed in connective tissue, and a highly homologous pseudogene, TNXA, flank CYP21A2 and CYP21A1P, respectively. Autosomal recessive tenascin X deficiency was described as a cause of Ehlers Danlos syndrome in 2001. We hypothesized that deletions of CYP21A2 might commonly extend into the TNXB gene, and we have been studying this phenomena in our Natural History Study.
The first evaluation of the potential clinical implications of TNXB heterozygosity in CAH patients was performed in our Natural History Study of CAH (www.ClinicalTrials.gov Identifier No. NCT00250159) at the NIH Clinical Center. In 2013, we prospectively studied 193 consecutive unrelated patients with CAH with clinical evaluations for manifestations of Ehlers Danlos syndrome and genetic evaluations for TNXB mutations. Heterozygosity for a TNXB deletion was present in 7% of CAH patients; these CAH patients were more likely than age-and sex-matched CAH patients with normal TNXB to have joint hypermobility, chronic joint pain, multiple joint dislocations, and a structural cardiac valve abnormality detected by echocardiography. Six of 13 probands had a cardiac abnormality, including the rare quadricuspid aortic valve, a left ventricular diverticulum, and an elongated anterior mitral valve leaflet. As a result of this study, the term CAH-X was coined to describe the subset of CAH patients who display an Ehlers Danlos syndrome phenotype resulting from to the monoallelic presence of a CYP21A2 deletion extending into the TNXB gene.
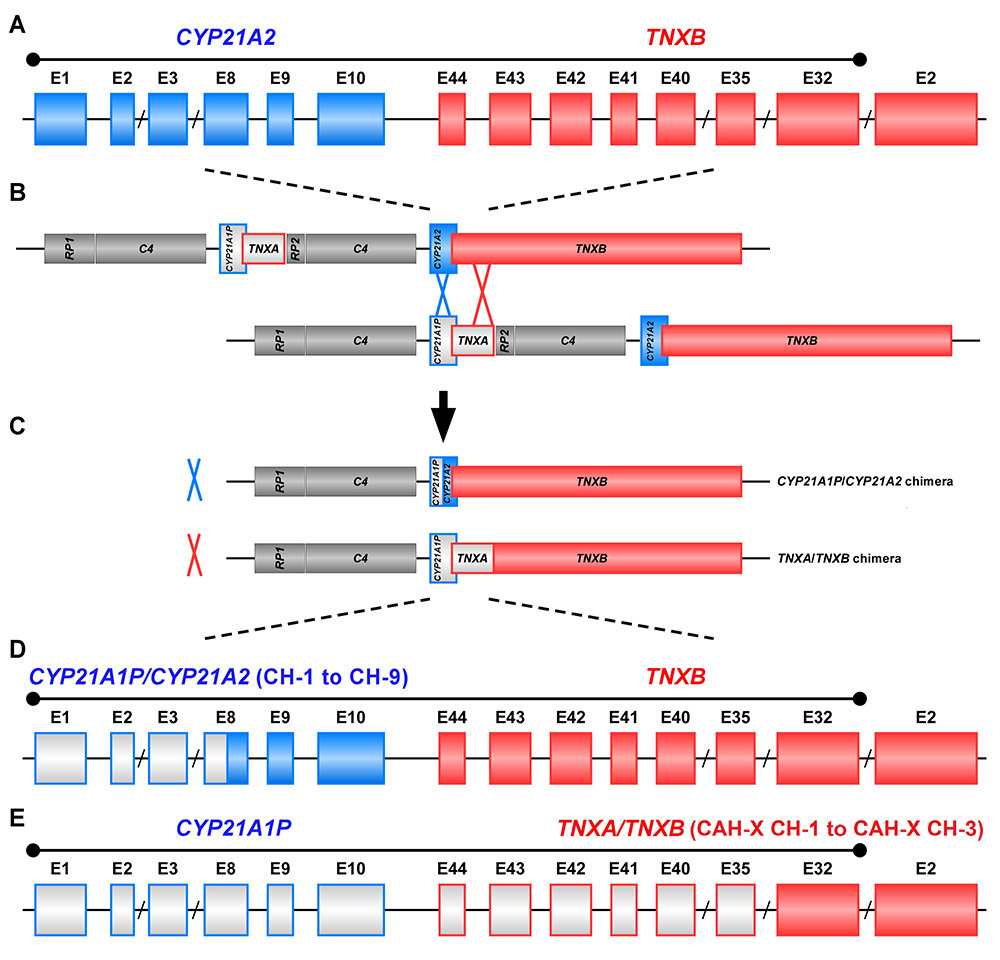
The study of CAH-X has provided insight into the recombination events that occur in the class III region of the major histocompatibility complex (MHC) locus. This region of the genome is predisposed to genetic recombination and misalignment during meiosis. The majority of deletions that occur generate chimeric CYP21A1P/CYP21A2 genes. Chimeric recombination between TNXB and TNXA also occurs (Figure 1). The recombination event deletes CYP21A2 and therefore represents a CAH disease–causing allele. We described three unique types of TNXA/TNXB chimera (CH): CAH-X CH-1 renders the gene nonfunctional, resulting in reduced dermal and serum TNX expression; CAH-X CH-2 alters protein structure; and CAH-X CH-3 is predicted to reduce protein folding energy. Our laboratory continues to investigate how TNXB contributes to the phenotype of CAH patients.
Click image to enlarge.
Figure 1. Schematic of CYP21A1P/CYP21A2 and TNXA/TNXB chimeric genes
Formation of chimeric genes occurs as a result of misalignment of homologous genes during meiosis. Active genes are in solid colors; pseudogenes are in grey and are framed with the color of the corresponding functional gene. Representative chimeric genes are shown. In total, there are nine known CYP21A1P/CYP21A2 chimeras (CH-1 to CH-9), and we identified three different types of TNXA/TNXB chimeras (CAH-X CH-1 to CAH-X CH-3) with different junction sites. Approximately 10 percent of patients with CAH due to 21-hydroxylase deficiency carry at least one TNXA/TNXB chimera, resulting in hypermobility-type Ehlers-Danlos syndrome or CAH-X syndrome.
To date, we have described 24 patients (19 families) with monoallelic CAH-X and three patients with biallelic CAH-X. Approximately 10 percent of patients with CAH owing to 21-hydroxylase deficiency are now estimated to be affected by CAH-X. Overall, CAH-X patients have generalized joint hypermobility, subluxations, and chronic arthralgia and about 25% have cardiac structural abnormalities. Patients with biallelic CAH-X show severe skin hyperextensibility with delayed wound healing and significant joint hypermobility. Other connective tissue disease manifestations in CAH-X patients include chronic tendonitis and/or bursitis, rectal prolapse, severe gastroesophageal reflux, and cardiac abnormalities.
The study of CAH-X syndrome provides insight into the complex clinical and genetic characteristics associated with CAH and promises to improve patient outcome through the development of focused medical management aimed at preventing long-term consequences.
Novel genetic causes of adrenal insufficiency
The most common cause of primary adrenal insufficiency in adults is autoimmunity, but a genetic etiology must be considered, especially in children. We studied four males from two unrelated families presenting with adrenal insufficiency in childhood, and we identified a genetic cause of their disease [Reference 2]. All had a nonclassic rare form of CAH resulting from a deficiency in the P450 cholesterol side-chain cleavage enzyme (P450scc), which is encoded by CYP11A1. All patients carried a CYP11A1 p.E314K variant. This previously reported variant was predicted to be benign by some models, highlighting the importance of carrying out functional studies. We showed that the p.E314K variant affects P450scc stability and its half-life and clinically impairs both adrenal and gonadal function. Our patients had normal male genitalia, and older males had normal pubertal progression with eventual evidence of peripubertal gonadal failure. Our study highlights the importance of performing genetic studies in all children diagnosed with primary adrenal insufficiency and suggests that mild defects in CYP11A1 may be common. Identifying the underlying cause of adrenal insufficiency is essential to providing appropriate clinical care.
Management of adrenal insufficiency
Patients with adrenal insufficiency are at risk for life-threatening salt-wasting adrenal crises. Management of illness episodes aims to prevent adrenal crises. This year, we evaluated rates of illnesses and associated factors in a large cohort of patients with adrenal insufficiency due to congenital adrenal hyperplasia followed prospectively at the NIH Clinical Center and receiving repeated glucocorticoid stress-dosing education. Also this year, longitudinal analysis of 156 CAH patients over 23 years was performed [Reference 3]. We evaluated a total of 2298 visits. During childhood there were more illness episodes and stress dosing than during adulthood; however, more emergency room visits and hospitalizations occurred during adulthood. The most robust predictors of stress dosing were young age, low hydrocortisone dose, and high fludrocortisone dose during childhood, and, during adulthood, female sex. Gastrointestinal and upper respiratory tract infections were the two most common precipitating events for adrenal crises and hospitalizations across all ages. Adrenal crisis with hypoglycemia occurred in 11 pediatric patients (ages 1.1–11.3 years). Undetectable epinephrine was associated with emergency room visits during childhood and illness episodes during adulthood. Based on this study, we revised our management of infectious illnesses for patients with adrenal insufficiency to include more frequent glucocorticoid dosing and recommendations regarding intake of simple and complex carbohydrates. Our new age-appropriate guidelines aim to reduce adrenal crises and prevent hypoglycemia, particularly in children.
New and improved biomarkers of CAH
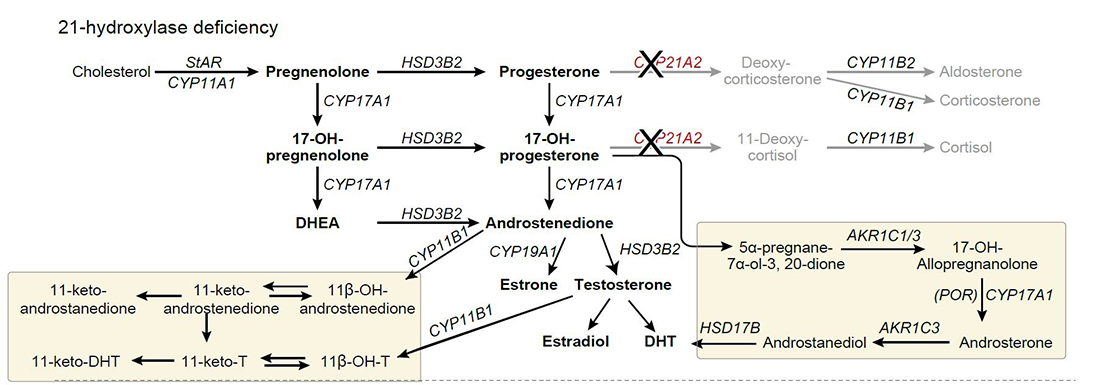
The diagnosis and management of CAH has been limited by inadequate biomarkers. Several pitfalls have been identified in the use of 17-hydroxyprogesterone, the most commonly used biomarker, for both diagnosis and management. In newborn screening, both false positives and false negatives are common. The development of liquid chromatography-tandem mass spectrometry (LC-MS/MS) panels of adrenal steroids has expanded the repertoire of potential new and improved steroid biomarkers. We found that steroids synthesized with the participation of 11β-hydroxylase (11-oxygenated C19 steroids) are abundant in patients with CAH resulting from 21-hydroxylase deficiency (Figure 2). With our collaborators, we examined the relationship between the serum steroid metabolome of children and adults with classic 21-hydroxylase deficiency and the presence of long-term disease complications. We found that elevations of these 11-oxygenated C19 steroids are associated enlarged adrenal glands and testicular tumors [Reference 4]. These newly described steroids may be useful in the diagnosis and management of CAH.
Click image to enlarge.
Figure 2. Classic and alternative steroidogenesis pathways leading to adrenal androgen production
In 21-hydroxylase deficiency, elevations of 17-hydroxyprogesterone and androstenedione can activate alternative steroidogenic pathways shown in yellow boxes. (El-Maouche D, Arlt W, Merke DP. Lancet 2017;390:2194).
Novel treatment approaches: circadian cortisol replacement
Humans have biological clocks with characteristic patterns of hormone secretion. Cortisol has a circadian rhythm, with levels low at sleep onset, rising between 0200hr and 0400hr, peaking in the early morning, and then declining throughout the day. Existing glucocorticoid replacement is non-physiologic, and the lack of diurnal rhythm may contribute to the many adverse outcomes observed in patients with adrenal insufficiency. In CAH, physiologic cortisol replacement might improve control of adrenal androgens at lower glucocorticoid doses, thus improving patient outcome. A promising treatment approach we are studying is physiologic cortisol replacement in patients with CAH.
In 2016, we successfully replaced cortisol in a physiologic manner through the use of a pump usually used to deliver insulin. A programmed 24-hour infusion of hydrocortisone was delivered subcutaneously for six months to eight patients with adrenal insufficiency due to 21-hydroxylase deficiency and with multiple co-morbidities. Following six months of pump therapy, patients experienced significant improvement in disease control at similar or lower daily doses of glucocorticoid, and significant improvement in quality-of-life and fatigue compared with oral conventional therapy. This year, we completed a long-term follow-up. Improvements achieved in androgen control, lean body mass, and health-related quality-of-life after six months of pump therapy were maintained at eighteen months [Reference 5].
Our group was the first to study circadian cortisol replacement in CAH patients with the use of a modified-release formulation of hydrocortisone, Chronocort® (CRADA #02800). We successfully completed a phase 2, open-label trial of 16 adults with classic CAH. Compared with various forms of conventional therapy prior to entry, six months of twice daily modified-release hydrocortisone yielded improved disease control throughout the day, using lower hydrocortisone dose equivalent. Successful completion of this phase 2 study (NCT 01735617), carried out at the NIH Clinical Center, resulted in a multi-center international phase 3, parallel arm, randomized, open-label study to determine whether this new modified-release preparation of hydrocortisone improves short-term clinical outcome. We are carrying out long-term follow-up to evaluate outcomes.
The studies provide insight into the role that circadian rhythm plays in the development of the co-morbidities associated with adrenal insufficiency. Physiologic cortisol replacement represents a novel treatment approach that promises to improve treatment outcome for patients with CAH, as well as other forms of adrenal insufficiency.
Novel treatment approaches: sex steroid blockade in children
As an alternative approach to the treatment of CAH, the effects of elevated androgen and estrogen could be prevented through the use of sex steroid blockade. Short-term (2–year) administration of an anti-androgen, aromatase inhibitor and reduced hydrocortisone was shown to normalize linear growth rate and bone maturation. A prospective long-term randomized parallel study to adult height of an antiandrogen (flutamide) and an aromatase inhibitor (letrozole), and reduced hydrocortisone dose vs. conventional treatment is near completion. The main outcome is adult height, and we will compare data compared between the treatment groups. The goal of this novel treatment approach is to normalize the growth and development of children with CAH and, ultimately, to determine whether this treatment regimen is effective in improving the growth of children with CAH. The Clinical Center is the ideal place to carry out such a long-term study of a rare disease.
Since the inception of our study of peripheral blockade of sex hormones using an antiandrogen and aromatase inhibitor, new and improved drugs that block sex steroids have been developed. In collaboration with extramural investigators, we are studying Abiraterone, an irreversible inhibitor of 17α-hydroxylase, a key enzyme required for testosterone synthesis, in a multicenter Phase 1/2 study in pre-pubescent children (NCT 02574910).
Additional Funding
- Cooperative Research and Development Agreement (CRADA) #02800 for Age-Appropriate Hydrocortisone Formulations for the Treatment of Adrenal Insufficiency including Congenital Adrenal Hyperplasia
- NIH U Grant: Abiraterone Acetate in Children with Classic 21-Hydroxylase Deficiency
Publications
- Hannah-Shmouni F, Morissette R, Sinai N, Elman M, Prezant TR, Chen W, Pulver A, Merke DP. Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in U.S. Ashkenazi Jews and Caucasians. Genet Med 2017;19:1276-1279.
- Kolli V, Kim H, Torky A, Lao Q, Tatsi C, Mallappa A, Merke DP. Characterization of the CYP11A1 non-synonymous variant p.E314K in children presenting with adrenal insufficiency. J Clin Endocrinol Metab 2018;104(2):269-276.
- El-Maouche D, Hargreaves CJ, Sinaii N, Mallappa A, Veeraraghavan P, Merke DP. Longitudinal assessment of illnesses, stress dosing and illness sequelae in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2018;103:2336-2345.
- Turcu AF, Mallappa A, Elman M, Avila NA, Marko J, Rao H, Tsodikov A, Auchus R, Merke DP. 11-Oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J Clin Endocrinol Metab 2017;102(8):2701-2710.
- Mallappa A, Nella AA, Sinaii N, Rao H, Gounden V, Perritt AF, Kumar P, Ling A, Liu CY, Soldin SJ, Merke DP. Long-term use of continuous subcutaneous hydrocortisone infusion therapy in patients with congenital adrenal hyperplasia. Clin Endocrinol 2018;89:399-407.
Collaborators
- Weibke Arlt, MD, Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, United Kingdom
- Richard J. Auchus, MD, PhD, University of Michigan, Ann Arbor, MI
- Veronica Gomez-Lobo, MD, Children's National Health System, Washington, DC
- James Marko, MD, Radiology and Imaging Sciences, NIH Clinical Center, Bethesda, MD
- Aikaterini A. Nella, MD, University of Texas Medical Branch, Galveston, TX
- Ann E. Pulver, ScD, The Johns Hopkins University School of Medicine, Baltimore, MD
- Martha Quezado, MD, Laboratory of Pathology, NCI, Bethesda, MD
- Richard J. Ross, MD, University of Sheffield, Sheffield, United Kingdom
- Ninet Sinaii, PhD, MPH, Biostatistics and Clinical Epidemiology Service, NIH Clinical Center, Bethesda, MD
- Steve J. Soldin, PhD, Department of Laboratory Medicine, NIH Clinical Center, Bethesda, MD
- Adina Turcu, MD, University of Michigan, Ann Arbor, MI
Contact
For more information, email dmerke@nih.gov or visit https://irp.nih.gov/pi/deborah-merke.


